Posted by John Staples • January 18th, 2012
Imagine, for a moment, that you recently received a brand new Time-O-Matic Time Machine as a gift. How far back would you have to set the dial to see that meaningful progress has been made in the treatment of hepatitis C?
First, let’s say you set the dial to January 2011 and –- Puff-kachunk! — you’re there. Standard treatment for chronic hepatitis C genotype 1 infection consisted of 48 weeks of pegylated interferon-alpha and ribavirin, an approach more likely to produce adverse effects than clearance of the virus.
Your next trip (Zing-kersplat!) takes you to May 2011. The oral serine protease inhibitors boceprevir and telaprevir have just become available for treatment of chronic HCV genotype 1 infection. When added to pegylated interferon and ribavirin, these direct-acting antivirals dramatically improve the rate of sustained virologic response (SVR). But many patients still do not respond to treatment, and medication intolerance remains an issue. You step into your Time-O-Matic and return to January 2012 feeling somewhat discouraged.
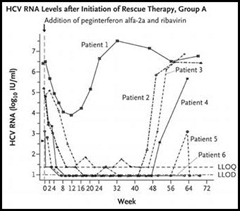
Fortunately, this week’s NEJM might raise your spirits. In it, Dr. Anna S. Lok (University of Michigan, Ann Arbor, MI) and colleagues report on an open-label, phase 2a study that used a combination of two direct-acting antivirals to treat chronic HCV genotype 1 infection. Twenty-one ‘null responders’ (patients who failed to achieve ≥2log10 decline in HCV RNA after ≥12 weeks of peginterferon and ribavirin) were randomized to 24 weeks of treatment in one of two treatment arms:
• Group A received BMS-790052 (an oral, first-in-class, NS5A replication complex inhibitor) and BMS-650032 (an oral NS3 protease inhibitor);
• Group B received BMS-790052 and BMS-650032 plus peginterferon alfa-2a and ribavirin.
Four of eleven (36%) patients in Group A and ten of ten (100%) of patients in Group B achieved an undetectable HCV RNA twelve weeks after completion of study treatment (SVR12, the primary end point). There were no deaths, serious adverse events, or discontinuations. The most common mild-to-moderate adverse effects were diarrhea, fatigue, headache, and nausea.
These results are exciting. The high rate of SVR12 achieved in Group B suggests that more effective treatment regimens may soon be available for null responders. But for editorialist Dr Raymond T. Chung (Massachusetts General Hospital, Boston, MA), it’s the Group A proof-of-concept results that really put HCV therapy “on the threshold of a treatment revolution.” The 36% of Group A patients achieving SVR12 demonstrate that combination direct-acting antivirals with non-overlapping resistance profiles can achieve HCV clearance without the use of interferon. If interferon and its adverse effects can be avoided, there is hope that treatment might be offered and accepted by a much greater number of patients.
“Current HCV treatment regimens are far from perfect,“ says primary care physician and NEJM deputy editor Dr. Mary Beth Hamel, “so it’s satisfying to see encouraging results from this kind of early-phase trial. It will be exciting to see what the future brings.”
Curious? Go ahead. Step into the Time-O-Matic and set the dial to see how far HCV therapy has progressed by January 2017. The rest of us, however, will just have to wait.
No comments:
Post a Comment