Where Is the Controversy?
Emily Dannhorn, James P O'Beirne
Future Virology. 2013;8(7):639-648.
Abstract and Introduction
Abstract
Liver transplantation (LT) is an accepted mode of treatment for patients with chronic liver disease. Historically, patients with HIV were excluded from LT programs, but with the introduction of highly effective antiretroviral regimens, HIV is no longer a contraindication. LT outcomes for some liver diseases in HIV-positive patients are equivalent to those observed in non-HIV-positive patients. This is not the case for patients coinfected with HIV and HCV, however, where results at 5 years have led to suggestions that LT for coinfection should be abandoned. This article examines the role of LT for HIV/HCV and identifies groups of patients where transplantation is associated with good outcomes. We believe that the application of existing knowledge to patient selection and organ allocation could improve outcomes further, and with the advent of directly acting antivirals for HCV, LT for HIV/HCV coinfection will no longer be controversial.
Introduction
Since the introduction of combined antiretroviral therapy (cART) in the mid-1990s, HIV has been a manageable disease for most, with an expected near-normal life expectancy.[1] Comorbidities, especially liver disease, are therefore of increasing clinical relevance, and chronic liver disease has become a leading cause of death in patients with HIV.[2,3] Liver transplantation (LT) was first performed in 1963 and has evolved from an experimental procedure to become the cornerstone of management of advanced end-stage liver disease and complications of cirrhosis.[4] Initially HIV infection was considered to be a contraindication to LT, but with the advent of cART, patients with HIV can now access LT and in most cases have outcomes similar to, if not better than, patients without HIV.[5] In HIV-infected individuals, HCV is a common cause of cirrhosis and there is therefore a need for LT in many individuals for this indication. Recently, however, due to poor results reported in the literature, the role of LT in patients coinfected with HIV and HCV has been called into question and some have suggested that LT for this indication should be abandoned.[6]
This is a regrettable situation given that LT is a surgical technique and specialty that has evolved through innovation and constant refinement of indications based on rigorous examination of outcomes. Many of the developments made in the field of LT were only made possible through continued experience gained from pioneering procedures. Without pushing these boundaries, we would not know whom to transplant (and whom not to transplant) with hepatocellular carcinoma (HCC) and acute liver failure, as well as others. To abandon LT for HIV/HCV-coinfected patients is, we believe, premature. In this article we hope to show that, using knowledge gained from recent experience, it is possible to select those coinfected patients who will do well, and with the advent of the directly acting antivirals (DAAs) for HCV, we hope to show not only that HIV/HCV coinfection has the potential to become a routine indication for LT, but also one with excellent results in the near future.
The Burden of Liver Disease in HIV Infection
Once regarded as a terminal disease, with the development of highly effective cART, HIV is now considered a chronic illness. Since its introduction in the 1990s, cART has significantly decreased rates of AIDS and AIDS-related deaths.[7] Half of the deaths occurring within patients established on cART regimens are not AIDS related. Liver disease is now the main non-AIDS cause of death in HIV-positive patients, having recently overtaken deaths due to cardiovascular disease. The vast majority of these deaths (over 90%) are associated with coinfection with chronic HCV and/or HBV.[8]
HIV is often associated with HCV and HBV coinfection due to comparable modes of transmission. Prevalence of coinfection with hepatitis C and HIV varies, but it is estimated that in the USA and Europe, up to a third of HIV-positive patients are coinfected (an estimated 300,000 individuals in the USA).[9] Rates of coinfection reaching up to 70% have been documented in eastern Europe, where the most common route of transmission is intravenous drug use.[10] Multiple infections (HIV, HBV and HCV) are most prevalent within intravenous drug users.
Due to the now near-normal life expectancy of individuals with HIV, other types of liver disease are also becoming important causes of morbidity and mortality, including drug-induced liver injury and nonalcoholic fatty liver disease.[3] These conditions also impact upon the numbers of coinfected patients developing end-stage liver disease. The number of patients with HIV and end-stage liver disease is increasing, and it is therefore clear that LT in HIV-infected patients is a partially unmet need, especially in coinfected patients, which urgently requires addressing.
LT for HIV/HCV Coinfection
Why the Controversy? The Increasing Burden of Disease in the Face of Limited Organ Availability
LT has been recommended as a treatment for patients "where the risk of death without a liver transplant is greater than the risk of death from transplantation", and where "transplantation is likely to result in a 50% chance of a >5-year survival with a quality of life acceptable to the patient".[11] A significant disparity exists between the number of patients requiring LT and the number of donated human livers, which inevitably leads to deaths on the waiting list. Decisions regarding listing and allocation of organs for LT are complex. In most systems, organs are allocated to patients with the most medical need (the sickest first), but attention also needs to be paid to the concept of utility; that is to say, patients who are likely to have the best outcome should receive priority. At present in the UK patients are accepted onto the waiting list if the severity of their liver disease (measured using the UK Model for End-Stage Liver Disease [UKELD] score) meets a certain level, defining the point at which the risk of dying from complications of LT is less than the 1-year risk of mortality from their liver disease (minimal listing criteria).[12] Each LT center maintains its own list and can internally prioritize candidates and allocate organs according to the center's wishes. While it is generally accepted that patients with worse liver disease will be transplanted first, this center-based allocation allows some degree of donor–recipient matching in an attempt to optimize outcomes for all.
Due to the demand for organs, the waiting time for LT in the UK is increasing. In 2012 the total number of patients active on the liver transplant list was 553; an increase of 8% from 2011. Over the last 10 years, there has been a steady increase in the number of patients registered on the active transplant waiting list, which has not been reflected by a similar increase in the number of available organs, resulting in an increase in death on the waiting list, which now stands at approximately 15–20%.
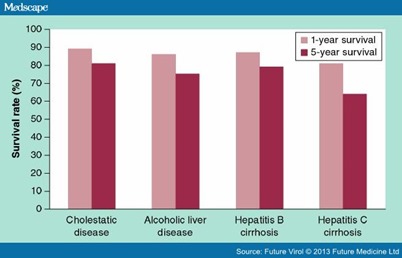
The problem of the gap between the need for LT and available organs is not unique to the UK and many organ allocation systems have been developed to try and reduce the deaths on the waiting list. One such system is the Model for End-Stage Liver Disease (MELD) system, which was introduced in the USA in 2002 and more recently in parts of Europe.[13] The MELD system allocates organs purely on the severity of liver disease, and while there is no doubt that its application has resulted in less waiting list mortality, there are concerns that this approach of transplanting the 'sickest first' has reduced medium- and long-term post-LT survival, effectively reducing the utility of LT.[14,15] The perfect allocation system would minimize deaths on the waiting list while optimizing long-term graft outcomes. Currently, such an allocation system does not exist, but it is acknowledged that the optimal balance between justice and utility is dependent on a number of donor and recipient factors. One such factor is the etiology of the patient's liver disease, which can have a major impact on expected survival following LT (Figure 1). As can be seen from Figure 1, transplantation for HCV-related cirrhosis is associated with inferior 1- and 5-year survival compared with other indications.[16] The reason for this is recurrent HCV infection in the graft, which is universal and results in progression to cirrhosis in up to 30% of patients after 5 years of follow-up. Although outcomes for transplanted HCV-monoinfected individuals are worse than other indications, the 5-year survival remains well above 50% and therefore the indication appears to justify LT on the basis of utility and is not controversial. This is not the case for HIV/HCV-coinfected patients, where relatively recent series have shown 5-year survival rates barely above 50%. It is for this reason that LT for HIV/HCV coinfection is questioned. At first glance it would seem acceptable to deny LT to patients on the basis of such poor outcomes, but it must be remembered that the cutoff of 50% 5-year survival as the basis for utility is purely arbitrary, and from an ethical standpoint it is questionable to deny LT on this factor alone. Furthermore, to deny LT to such patients is to reject the opportunity to learn from past experience and apply those lessons for the benefit of future patients. It may well be that HIV/HCV-coinfected patients need different selection criteria or organ allocation systems in order to maximize benefit from LT. Successful progress in this regard rests on continuing to transplant coinfected patients in order to understand the differences between HCV-coinfected and -monoinfected patients with respect to both pre- and post-transplant variables in order to identify areas where patient selection, organ allocation or post-transplant treatment can be improved.
Figure 1.
The 1- and 5-year survival rates post-liver transplantation for common causes of cirrhosis. Even in the absence of coinfection with HIV, the 5-year survival rate in patients with HCV is inferior due to recurrent disease.Adapted with permission from [16].
Why is Coinfection Different From Monoinfection?
The exact effects of HIV on hepatitis C remain unclear, but it is accepted that HIV accelerates both HBV and HCV liver disease, more so with worsening of the HIV-associated immunodeficiency.[9] The combination of HIV/HCV coinfection is associated with a reduced rate of spontaneous HCV RNA clearance and therefore an increased likelihood of developing chronic HCV.[17] With established HCV infection, there follows a more rapid rate of fibrosis progression.[18] Predictors of fibrosis progression include detectable HIV viral loads, low CD4+ cell counts, the level of baseline necroinflammatory activity on liver biopsy and increased alcohol consumption (>50 g/day).[19] Compared with monoinfected patients, coinfected patients have a more rapid course of fibrosis. In paired liver biopsy studies, significant fibrosis progression was seen in 25% of coinfected patients over 3 years compared with 12% of monoinfected patients over a similar time period.[20] Following development of cirrhosis, the course of the liver disease is accelerated. Indeed, in coinfected patients with established cirrhosis, the median estimated survival time is only 13 months following the first decompensating event (e.g., bleeding varices, encephalopathy, renal failure). Interestingly, and very relevant for the issue of LT in HIV/HCV coinfection, the risk of death following decompensation may not be accurately reflected by the MELD score, which may disadvantage coinfected patients in organ allocation systems based on MELD.[21] HIV also increases the risk of development of HCC. Recent studies have shown that the incidence of HCC in predominantly HCV-coinfected patients is increasing, with studies from Spain and the USA showing a 10–23-fold increase in incidence over the last 10 years.[22–24]
It can be seen from the evidence above that liver disease in HIV/HCV-coinfected patients is a much more aggressive entity than that seen in monoinfected cohorts, with a more rapid clinical course, especially after the development of cirrhosis. Clearly, therefore, the best approach to this problem would be to identify and treat patients with HCV prior to the development of advanced fibrosis. There are a number of factors that would limit the success of this strategy. First, the majority of HCV infections in HIV-positive patients are HCV genotype 1 and therefore more difficult to treat.[10] With the current standard of care (pegylated interferon and ribavarin), sustained virological response rates are poor (15–30%) and treatment is difficult to tolerate. Additionally, HCV treatment uptake remains poor, especially among injecting drug users, which further reduces the number of patients who may benefit.[25,26] Second, while the advent of DAAs for HCV offers some hope for the future, only limited experience in coinfected patients has been published thus far. Current triple therapy regimens using the protease inhibitors telaprevir or boceprevir have been used in selected patients with coinfection in two Phase II trials.[27,28] Notwithstanding the potential for drug interactions with antiretrovirals, increased sustained virological response were seen compared with patients treated with standard of care (60–74 vs 26–44%), but it must be remembered that these patients were highly selected and therefore these results are unlikely to be applicable to the majority of patients with advanced fibrosis or cirrhosis.[26] Therefore, despite this advance in therapy, there is likely to be a need for LT in these patients for some time to come.
LT Outcomes in HIV/HCV Coinfection: From Past to Present
As previously mentioned, HIV was considered to be a contraindication to LT in the pre-cART era. However with the increasing realization that HIV could be controlled, and experience in a small number of patients who had been transplanted and then subsequently found to be seropositive, the issue of LT in HIV-positive recipients was revisited. The King's College Group published one of the earliest series in 2001.[29] This small series of five patients was one of the first to examine outcomes of patients known to be HIV-positive at the time of LT. Of the five patients described, three were coinfected with HCV. ART was used in all patients, three pre-LT and two post-LT. The outcomes for the HCV patients were poor, with all three patients dying within 16 months of LT from a severe form of HCV recurrence in the graft (fibrosing cholestatic hepatitis). It is notable that two patients received antiviral treatment with interferon and ribavarin, one receiving treatment as early as 2 weeks, and all the HCV patients had at least one episode of rejection. This report does not describe in detail the interaction between the cART and immunosuppressive drugs, but it is interesting that at the time of death the CD4 count ranged from 5 to 87 cells/mm3, suggesting that there had been some loss of HIV control during the postoperative period, perhaps due to difficulty with drug interactions. The non-HCV-infected patients in the study did extremely well and remained free from complications related to the liver graft or HIV. This finding was considered encouraging and more centers began to transplant HIV-positive patients, especially after publication of guidelines in the UK ( Box 1 ).[30]
Box 1. UK guidelines for consideration of liver transplantation in HIV infection (in addition to the usual indications and contraindications).
Meets conventional criteria for listing for liver transplantation † and:
|
† UK Model for End-Stage Liver Disease score >49, diuretic-resistant ascites or other variant syndrome, hepatic encephalopathy or hepatocellular carcinoma within accepted criteria.
‡ Except in de novo presentation of HIV infection in cases of acute liver failure.
Adapted with permission from [30].
Shortly following the publication of the initial series from King's, Ragni et al. published their experience of transplantation in HIV-infected individuals.[31] They were able to show cumulative survival among 24 HIV-positive HAART recipients that was similar to age- and race-matched HIV-negative recipients. At 12, 24 and 36 months after LT, respective estimated survival rates were 87, 73 and 73% among HIV-positive patients and 87, 82 and 78% among HIV-negative patients, confirming that transplantation was feasible and effective in HIV infection. Similar to the King's group they found that survival in patients with HIV/HCV coinfection was significantly lower than in HCV controls.
A number of retrospective and prospective series have now confirmed that outcomes in HIV/HCV coinfection are inferior to other indications, especially under longer periods of follow-up.
Table 1 summarizes patient survival at set intervals as reported in larger series in the cART era to date. One striking feature of the series published so far is a wide variation in short- and medium-term outcomes, especially in smaller series. This raises the question as to whether there is significant variation in center practice that is impacting on outcome. The more recent series involved larger number of patients enrolled in multiple centers prospectively, thus allowing more detailed analysis of variables that may be associated with outcome.
Table 1. The 1–, 3– and 5-year survival rates in published series of liver transplantation for HIV/HCV coinfection.
| Study (year) | n | 1-year survival (%) | 3-year survival (%) | 5-year survival (%) | Ref. |
|---|---|---|---|---|---|
| Ragni et al. (2003) | 15 | 80 | 57 | – | [31] |
| de Vera et al. (2006) | 27 | 67 | 56 | 33 | [49] |
| Vennarecci et al. (2007) | 12 | 83 | 58 | – | [50] |
| Duclos-Vallée et al. (2008) | 35 | – | 73 | 51 | [51] |
| Terrault et al. (2012) | 89 | 76 | 60 | – | [35] |
| Miro et al. (2012) | 84 | 88 | – | 54 | [32] |
| Baccarani et al. (2012) | 26 | – | 78 | 68 | [52] |
LT for HIV/HCV Coinfection: A Work in Progress?
Although not necessarily demonstrating improved results, more recent multicenter reports have been able to identify a number of patients who could potentially make up a subgroup of HIV/HCV-coinfected individuals with improved graft survival and mortality rates after LT. Miro et al. carried out a prospective multicenter cohort trial examining the outcomes of 84 HIV/HCV-coinfected patients and 252 matched HCV-monoinfected patients undergoing LT between 2002 and 2006.[32] The majority of patients were HCV genotype 1. They demonstrated that LT was an effective short-term procedure in both mono- and co-infected patients, with similar 1-year survival rates (88 vs 90%). However, following longer periods of time, survival in the coinfected group was significantly lower (5-year survival: 54 vs 71%; p = 0.008). HIV was identified as an independent predictor of death in patients with HCV.
Other than survival, there were additional important differences between the two groups. For instance, biopsy-proven acute cellular rejection was much more common in the coinfected group (38 vs 20%; p < 0.001). The cause of this is speculative, but presumably HIV-linked immunomodulation and problems achieving satisfactory immunosuppressive levels due to drug interactions are operative. The increased rates of rejection and its subsequent treatment observed in the coinfected group could be responsible for more severe HCV recurrence, as is seen in studies of monoinfection and LT.[33,34] In multivariate analysis, other factors relevant to mortality in the coinfected group were MELD score pretransplantation, LT performed at a center with low activity (at centers performing <1 transplant/year, mortality was almost three-times higher) and infection with HCV genotype 1. Having a negative HCV RNA viral load at any time (pre- or post-LT) had a positive impact on mortality. As previously established, factors pertaining to the donor (e.g., donor risk index) also correlated with survival.
A similar study published by Terrault et al. allows further analysis of factors associated with poor outcome.[35] This study was prospective and collected data from multiple centers in the USA, comparing patient and graft survival for 89 HIV/HCV-coinfected patients and two control groups: 235 patients with HCV monoinfection and all US transplant recipients over the age of 65 years between 2003 and 2010.[35] Eight of the coinfected cohort underwent combined liver–kidney transplantation. Patients in the study underwent transplantation at 17 different centers across the USA.
The results demonstrated that HIV/HCV patients were younger at transplant than their HCV controls, and had a lower BMI at listing. They were also more frequently coinfected with HBV. Coinfected recipients also received a higher proportion of organs from donation after circulatory death (DCD) donors, and had longer warm ischemic times – factors known to be related to an increased risk of graft loss.[36] Immunosuppressive regimens in the coinfected group were less likely to involve tacrolimus-based agents in the initial stages. The 1- and 3-year survival rates were 76 and 60% for HIV/HCV-coinfected patients, and 92 and 79% for HCV-monoinfected patients (p < 0.001). Graft loss due to infection or multiorgan failure was observed more frequently in the coinfected group; graft loss due to malignancy was seen more in the monoinfected cohort. Of note, there were no deaths due to infections related to HIV, and a history of AIDS-related pathology pretransplant was not associated with worsened survival post-transplantation.
In the multivariate analysis, HIV was the only factor associated with a significantly increased risk of death. In the coinfected group, undergoing combined liver–kidney transplantation was associated with increased mortality. As with the results from Miro et al.,[32] a lower BMI (BMI <21 at listing) was a predictor of increased mortality, as was receiving an organ from an non-HCV-positive donor, or an older age donor. When coinfected patients without these 'high-risk factors' were analyzed, the outcomes in terms of postoperative graft and patient survival were similar to those in the >65 years of age control group.
Increased rates of rejection were also demonstrated in the coinfected group compared with the HCV-monoinfected group in this study. The incidence of rejection over a 3-year period was 39% in the coinfected group versus 24% in the HCV-monoinfected patients; in excess of 50% of episodes occurred within the first 21 days post-LT in the HIV/HCV group. HIV was the only factor significantly associated with rejection.
Recent Data: What Have We Learnt?
The two recent large studies outlined above have identified a number of pre- and peri-transplant variables that impact on mortality, and have suggested a possible subgroup of coinfected patients that, in the absence of these high-risk features, can do well with LT (Box 2).
Box 2. Pretransplant and donor variables associated with increased risk of graft loss and mortality in coinfected liver transplant recipients.
Pretransplant/recipient variables
|
Donor variables
|
† Reflecting renal dysfunction in the recipient.
The detailed analysis afforded by these studies gives interesting insight into potential areas of improvement in the selection of LT recipients and their subsequent management.
Coinfected patients on the LT waiting list often have lower BMIs at the time of transplant compared with monoinfected controls. There are many potential reasons for this observation, but as malnutrition is a common feature of advanced liver disease and has prognostic importance, it is possible that the coinfected patients had more advanced liver disease at the time of listing, the severity of which was not reflected by the MELD score, as has previously been described.[21,37] BMI is a reproducible and reliable marker, which could be incorporated into future selection criteria for this cohort of patients in order to improve prognostication over the MELD score alone. Following on from this, it seems logical that patients at risk of malnutrition should receive aggressive nutritional support in order to maintain BMI >21. This would be facilitated by early referral to the transplant center for patients who are losing weight independently of the MELD score. The importance of renal function is highlighted by the outcomes reported in the Terrault et al. study.[35] Combined liver–kidney transplantation is utilized in patients with renal dysfunction expected not to recover following LT. The poor outcome in those patients receiving combined grafts as well as recent data showing worse renal function post-LT in coinfected patients receiving a liver alone suggest that renal impairment is an ominous prognostic sign in potential LT recipients, and highlights once again the importance of early referral. This finding is of major relevance to coinfected patients seeking LT in systems that use the MELD allocation system. As creatinine is a component of the MELD score, patients with renal dysfunction will have a higher MELD score and therefore are more likely to be transplanted. For the coinfected patient this means that they will only have a chance of being transplanted when they are at risk of a poor outcome due to renal impairment.
Donor selection has also been reviewed, and several factors affecting mortality and graft loss have been described. For patients with coinfection the use of non-HCV-positive donors was associated with increased rates of graft loss; a finding not seen in patients post-LT with HCV monoinfection. This finding is unexplained and merits further work, as these grafts are a valuable source of organs in the era of organ shortage. The finding of increased rates of graft loss in patients receiving livers from older donors is perhaps unsurprising as this is a recognized risk factor for severe HCV recurrence in monoinfected patients.[38] DCD donors are associated with increased risk of primary nonfunction and biliary complications.[39,40] The effect of DCD on the severity of HCV recurrence is debated, but it may be relevant in coinfected patients, given that this was a significant factor for graft loss in the multivariate analysis.[41,42] DCD donors represent an important and increasing source of grafts, and so to suggest that DCD donors should be avoided for coinfected recipients risks prejudicing their outcome by increasing the risk of dying on the waiting list while waiting for a liver. Certainly it seems logical that if DCD doses are used in coinfected patients they should be only those from younger donors with shorter ischemic times.
Higher rates of cellular rejection were also described in both of the aforementioned recent studies, which were associated with worse HCV recurrence. Although the definite reasons for this have yet to be ascertained, increased rejection has been attributed to the known immunomodulatory effects of HIV, as well as the difficulties in achieving optimal immunosuppression post-LT.[43] Treatment for rejection has been identified as a risk factor for severe HCV recurrence and graft loss, emphasizing the importance of preventing acute rejection in the coinfected cohort.[33] It has been suggested that overly cautious immunosuppressive use post-LT in these patients, due to concerns about worsening HIV-related diseases, has resulted in increased episodes of rejection. Similarly, the complex drug interactions have made reaching optimal levels of immunosuppression very challenging.[44]
The major issue with medication after LT in HIV/HCV-coinfected patients is the complex range of drug interactions between cART and immunosuppressive drugs. The majority of immunosuppressive medications used are metabolized by the cytochrome P450 system. Protease inhibitors, including ritonavir, can reduce the metabolism of calcineurin inhibitors, such as cyclosporine and tacrolimus, which make up the mainstay of immunosuppression regimens post-LT. The therapeutic range for tacrolimus is narrow and significant side effects such as nephrotoxicity and neurotoxicity often occur at levels above >15 ng/ml.[45] The usual therapeutic range for tacrolimus is 5–10 ng/ml, which is close to the toxic level; therefore, medications that could increase tacrolimus levels should be used with caution. Combined prescription of tacrolimus and ritonavir-boosted PI regimens requires a marked decrease in dose of tacrolimus and close drug monitoring to avoid toxicity. Published case reports have used single doses of 0.5–1 mg of tacrolimus every 1–3 weeks when combined with ritonavir-containing cART, and patients have required frequent therapeutic drug monitoring to maintain constant drug levels within the therapeutic range.[46] This is very challenging to maintain in clinical practice, and while this approach is successful in preventing overt toxicity, less is known about the actual drug exposure during the extended dosing period, leading to concerns that patients may be underimmunosuppressed. The complex interplay between levels of immunosuppression, maintaining HIV negativity with cART and avoiding toxicity is a challenge, and it is perhaps no surprise that center experience is a major factor in transplant outcome. In fact, mortality is almost three-times higher in coinfected patients undergoing LT at centers with low transplant activity (<1 LT/year for HIV) compared with more experienced centers.[32]
Miro et al. factored center experience into a risk score for mortality using other pretransplant variables using the formula: Exp([0.81966 if genotype = 1] + [0.05748 MELD pre-LT] + [1.03540 if center does <1 orthotopic LT in HIV-infected patients/year]).[32] A risk score cutoff of 1.07795 allowed classification of the 84 LT recipients as having a low risk or a high risk of death. Sixty patients in the study were calculated to be low risk and 5-year survival in this cohort was excellent, at 70%.
Conclusion
With the advances made in cART for the management of HIV, increasing numbers of patients are presenting with end-stage liver disease, commonly due to coinfection with HCV. After transplantation, the outcomes for this cohort have consistently been worse when compared with those for HCV-monoinfected patients. Rather than abandon this indication for LT, the liver transplant community needs to respond to the challenge and use the currently available evidence to revise the selection criteria and allocation policies for this group of patients. It is already possible to define a group of patients who will do as well as other non-HIV-infected LT recipients, and simple changes to the selection of candidates for LT will improve outcomes further. Due to the use of antivirals, LT outcomes for patients transplanted for HBV/HIV coinfection are excellent and the advent of the DAAs to treat HCV offers the very real possibility that HIV/HCV will very quickly become an excellent indication for LT.
Future Perspective
There is no doubt that the current medium- to long-term outcomes of LT in coinfected patients are unacceptable. To not use the published data to change the selection, allocation criteria and post-transplant management of coinfected patients runs the risk of this indication being abandoned due to the competing needs of non-coinfected patients who may expect better post-LT outcomes. What needs to be done? Firstly, using the published data it is already possible to identify a subgroup of patients who will do well with LT (Box 3), especially in an experienced center. LT in these patients should not be controversial and outcomes can be expected to be excellent. However, patients with this phenotype are not prioritized effectively within organ allocation systems designed around liver disease severity, and this suggests that coinfected patients should have a different allocation system or be given priority based on MELD combined with other criteria such as BMI. These changes will be hard to enact in the current era of donor shortage and are perhaps unachievable. A more realistic aim is to restrict LT in coinfected patients with renal dysfunction or where the BMI is less than 21. This simple change will improve outcome. Early referral to a LT center is key to ensuring timely access to LT prior to the development of these complications. As previously mentioned, current organ allocation systems do not necessarily favor coinfected patients with the optimal attributes for good post-LT outcomes. To avoid excessive waiting times, patients should be encouraged to seek other sources of organs, such as live and domino donation, in an attempt to shorten waiting times. Where possible, the use of marginal (older, steatotic) grafts should be avoided in coinfected recipients, and the use of HCV-positive grafts should be cautious until more is known about the outcomes of LT using these organs. The major problem post-LT is HCV recurrence, which is the most common cause of graft loss. HCV recurrence is associated with steroid boluses to treat rejection episodes, and it is disappointing that rejection appears to be more frequent in coinfected recipients. Addressing the issue of rejection will decrease HCV recurrence and improve outcomes. This goal is achievable as we understand more about the interactions between calcineurin inhibitors and cART and become more adept at individualized immunosuppression protocols. Newer antiviral regimens without the use of protease inhibitors should facilitate the easier dosing of immunosuppression and lead to less rejection. The complexities of immunosuppression dosing and cART management highlight the importance of an experienced multidisciplinary team in the management of these patients. Such teams will only exist in larger centers, and given the association of center volume with mortality, it is arguable that this activity should only occur in centers transplanting regularly for this indication.
Box 3. Characteristics of HIV/HCV-coinfected patients associated with good outcomes following liver transplantation.
|
MELD: Model for End-Stage Liver Disease.
The new era of DAAs offers encouragement for the future in terms of treating patients both before and after transplantation. At the present time, the currently available protease inhibitors (telaprevir and boceprevir) are only active against genotype 1 infection, and drug interactions with cART and CNIs will limit their applicability in the post-transplant setting. Other DAAs will shortly be available, and case reports of successful treatment of post-LT HCV recurrence with drugs such as daclatasvir and sofosbuvir – albeit in a non-HIV setting – show promise for the future.[47,48]
References
-
Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet372(9635),293–299 (2008).
-
Castellares C, Barreiro P, Martín-Carbonero L et al. Liver cirrhosis in HIV-infected patients: prevalence, aetiology and clinical outcome. J. Viral Hepat.15(3),165–172 (2008).
-
Joshi D, O'Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with HIV infection. Lancet377(9772),1198–1209 (2011).
• Excellent review of liver-related complications in HIV infection. -
Starzl TE, Demetris AJ, Thiel DV. Liver transplantation. N. Engl. J. Med.321,1014–1022 (1989).
-
Fox AN, Vagefi PA, Stock PG. Liver transplantation in HIV patients. Semin. Liver Dis.32(2),177–185 (2012).
-
Joshi D, Aluvihare V, Belgaumkar A et al. Liver transplantation for HIV: analysis of outcomes suggest HIV/HCV co-infected patients have prohibitively poor survival at 5 years. Hepatology48(Suppl. 1),311 (2008).
-
Mocroft A, Brettle R, Kirk O et al. Changes in the cause of death among HIV positive subjects across Europe: results from the EuroSIDA study. AIDS16(12),1663–1671 (2002).
-
Weber R, Sabin CA, Friis-Moller N et al. Liver-related deaths in persons infected with the human immunodeficiency virus. Arch. Intern. Med.166,1–10 (2006).
-
Lacombe K, Rockstroh J. HIV and viral hepatitis coinfections: advances and challenges. Gut61(Suppl. 1),i47–i58 (2012).
-
Soriano V, Mocroft A, Rockstroh J et al. Spontaneous viral clearance, viral load, and genotype distribution of hepatitis C virus (HCV) in HIV-infected patients with anti-HCV antibodies in Europe. J. Infect. Dis.28,1337–1344 (2006).
-
Neuberger J, James O. Guidelines for selection of patients for liver transplantation in the era of donor-organ shortage. Lancet354(9190),1636–1639 (1999).
• Review article explaining the rationale behind the 50% 5-year survival concept. -
Neuberger J, Gimson A, Davies M et al. Selection of patients for liver transplantation and allocation of donated livers in the UK. Gut57(2),252–257 (2008).
• Review article outlining minimal listing criteria using the UK Model for End-Stage Liver Disease (UKELD) and the current organ allocation system in the UK. -
Wiesner RH, McDiarmid SV, Kamath PS. MELD and PELD: application of survival models to liver allocation. Liver7(7),567–580 (2001).
-
Locke JE, Warren DS, Singer AL, Segev DL. Declining outcomes in simultaneous liver-kidney transplantation in the MELD era: ineffective usage of renal allografts. Transplantation85(7),935–942 (2008).
-
Benckert C, Quante M, Thelen A. Impact of the MELD allocation after its implementation in liver transplantation. Scand. J. Gastroenterol.46,941–948 (2011).
-
Adam R, Karam V, Delvart V et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J. Hepatol.57(3),675–688 (2012).
-
Graham CS, Baden LR, Yu E et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin. Infect. Dis.33(4),562–569 (2001).
-
Bonnard P, Lescure FX, Amiel C et al. Documented rapid course of hepatic fibrosis between two biopsies in patients coinfected by HIV and HCV despite high CD4 cell count. J. Viral Hepat.14(11),806–811 (2007).
-
Benhamou Y, Bochet M, Di Martino V, Charlotte F. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. Hepatology30(4),1054–1058 (1999).
-
Sulkowski MS, Mehta SH, Torbenson MS et al. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS21(16),2209–2216 (2007).
-
Merchante N, Girón-González JA, González-Serrano M et al. Survival and prognostic factors of HIV-infected patients with HCV-related end-stage liver disease. AIDS20(1),49–57 (2006).
-
Ioannou GN, Bryson CL, Weiss NS, Miller R, Scott JD, Boyko EJ. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology57(1),249–257 (2013).
-
Puoti M, Rossotti R, Garlaschelli A, Bruno R. Hepatocellular carcinoma in HIV hepatitis C virus. Curr. Opin. HIV AIDS6(6),534–538 (2011).
-
Merchante N, Merino E, López-Aldeguer J et al. Increasing incidence of hepatocellular carcinoma in HIV-infected patients in Spain. Clin. Infect. Dis.56(1),143–150 (2013).
-
Grebely J, Oser M, Taylor LE, Dore GJ. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. J. Infect. Dis.207(Suppl. 1),S19–S25 (2013).
-
Sulkowski MS. HCV therapy in HIV-infected patients. Liver Int.33(Suppl. 1),63–67 (2013).
-
Sulkowski M, Pol S, Cooper C et al. Boceprevir plus peginterferon/ribavirin for the treatment of HCV/HIV co-infected patients: interim on-treatment results. Presented at: 49th Annual Meeting of the Infectious Diseases Society of America. Boston, MA, USA, 20–23 October 2011.
-
Sherman KE, Rockstroh JK, Dieterich DT. Telaprevir in combination with peginterferon alfa 2a/ribavirin in HCV/HIV coinfected patients: a 24-week Treatment interim analysis [abstract LB-8]. Presented at: 62nd Annual Meeting of AASLD. San Francisco, CA, USA, 4–8 November 2011.
-
Prachalias AA, Pozniak A, Taylor C, Srinivasan P. Liver transplantation in adults coinfected with HIV. Transplantation72(10),1684–1688 (2001).
•• First reported series of transplanted HIV-infected patients. -
O'Grady J, Taylor C, Brook G. Guidelines for liver transplantation in patients with HIV infection. HIV Med.6(2),149–153 (2005).
•• Current UK guidelines for transplantation in HIV-infected patients in the UK. -
Ragni MV, Belle SH, Im KA, Neff G. Survival of human immunodeficiency virus-infected liver transplant recipients. J. Infect. Dis.188(10),1412–1420 (2003).
-
Miro JM, Montejo M, Castells L et al. Outcome of HCV/HIV-coinfected liver transplant recipients: a prospective and multicenter cohort study. Am. J. Transplant.12(7),1866–1876 (2012).
• Multicentric prospective study of outcomes in HIV/HCV coinfection. -
Sheiner PA, Schwartz ME, Mor E, Schluger LK. Severe or multiple rejection episodes are associated with early recurrence of hepatitis C after orthotopic liver transplantation. Hepatology21(1),30–34 (1995).
-
Samonakis DN, Germani G, Burroughs AK. Immunosuppression and HCV recurrence after liver transplantation. J. Hepatol.56(4),973–983 (2012).
-
Terrault NA, Roland ME, Schiano T et al. Outcomes of liver transplant recipients with hepatitis C and human immunodeficiency virus coinfection. Liver Transpl.18(6),716–726 (2012).
•• Large multicenter evaluation of predictors of outcome in HIV/HCV coinfection. -
Grewal HP, Willingham DL, Nguyen J et al. Liver transplantation using controlled donation after cardiac death donors: an analysis of a large single-center experience. Liver Transpl.15(9),1028–1035 (2009).
-
Merli M, Giusto M, Gentili F, Novelli G. Nutritional status: its influence on the outcome of patients undergoing liver transplantation. Liver Int.30(2),208–214 (2010).
-
Wali M, Harrison RF, Gow PJ, Mutimer D. Advancing donor liver age and rapid fibrosis progression following transplantation for hepatitis C. Gut51(2),248–252 (2002).
-
Maheshwari A, Maley W, Li Z, Thuluvath PJ. Biliary complications and outcomes of liver transplantation from donors after cardiac death. Liver Transpl.13(12),1645–1653 (2007).
-
Pine JK, Aldouri A, Young AL et al. Liver transplantation following donation after cardiac death: an analysis using matched pairs. Liver Transpl.15(9),1072–1082 (2009).
-
Tao R, Ruppert K, Cruz RJ et al. Hepatitis C recurrence is not adversely affected by the use of donation after cardiac death liver allografts. Liver Transpl.16(11),1288–1295 (2010).
-
Hernandez-Alejandro R, Croome KP, Quan D et al. Increased risk of severe recurrence of hepatitis C virus in liver transplant recipients of donation after cardiac death allografts. Transplantation92(6),686–689 (2011).
-
Gow PJ, Pillay D, Mutimer D. Solid organ transplantation in patients with HIV infection. Transplantation72(2),177–181 (2001).
-
van Maarseveen EM, Rogers CC, Trofe-Clark J, van Zuilen AD, Mudrikova T. Drug–drug interactions between antiretroviral and immunosuppressive agents in HIV-infected patients after solid organ transplantation: a review. AIDS Patient Care STDS26(10),568–581 (2012).
-
Bäckman L, Nicar M, Levy M et al. FK506 trough levels in whole blood and plasma in liver transplant recipients. Correlation with clinical events and side effects. Transplantation57(4),519–525 (1994).
-
Bickel M, Anadol E, Vogel M et al. Daily dosing of tacrolimus in patients treated with HIV-1 therapy containing a ritonavir-boosted protease inhibitor or raltegravir. J. Antimicrob Chemother.65(5),999–1004 (2010).
-
Fontana RJ, Hughes EA, Appelman H, Hindes R, Dimitrova D, Bifano M. Case report of successful peginterferon, ribavirin, and daclatasvir therapy for recurrent cholestatic hepatitis C after liver retransplantation. Liver Transpl.18(9),1053–1059 (2012).
-
Fontana RJ, Hughes EA, Bifano M et al. Sofosbuvir and daclatasvir combination therapy in a liver transplant recipient with severe recurrent cholestatic hepatitis C. Am. J. Transplant.13(6),1601–1905 (2013).
• First report of successful non-interferon-based treatment of severe HCV recurrence post-transplant. -
de Vera ME, Dvorchik I, Tom K et al. Survival of liver transplant patients coinfected with HIV and HCV is adversely impacted by recurrent hepatitis C. Am. J. Transplant.6(12),2983–2993 (2006).
-
Vennarecci G, Ettorre GM, Antonini M et al. Liver transplantation in HIV-positive patients. Transplant. Proc.39(6),1936–1938 (2007).
-
Duclos-Vallée JC, Féray C, Sebagh M et al. Survival and recurrence of hepatitis C after liver transplantation in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology47(2),407–417 (2008).
-
Baccarani U, Scudeller L, Adani GL, Viale P, Tavio M. Is liver transplantation feasible in patients coinfected with human immunodeficiency virus and hepatitis C virus? Liver Transpl.18(6),744–745; author reply 746 (2012).
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
No comments:
Post a Comment