Hawaii J Med Public Health. 2013 September; 72(9 Suppl 4): 6–13.
PMCID: PMC3764547
Matthew J Akiyama, MD, MSc, Joy I Piotrowski, Marina M Roytman, MD, Siu MA Chan, FNP-BC, Leena K Hong, PA-C, Leslie Huddleston, PA-C, RN, Ruby Trujillo, APRN, and Naoky CS Tsai, MD
Abstract
Recent advances in treatment of chronic hepatitis C virus have improved significantly due to the introduction of two new protease inhibitors—telaprevir and boceprevir. In combination with the previous standard of care, peginterferon and ribavirin, telaprevir and boceprevir have demonstrated improved sustained virologic response rates for HCV genotype 1 patients by approximately 30%. The purpose of this study was to assess the validity of large clinical trial data with respect to efficacy and side effects in a community setting in Honolulu, Hawai‘i. This retrospective study was performed by reviewing the charts of 59 chronic HCV patients who were started on triple therapy from July 1, 2011 to July 7, 2012. Sustained virologic response was attained by 73% of patients treated with telaprevir and 46% of patients treated with boceprevir respectively. Our clinical experience with telaprevir demonstrates that SVR rates are compatible with published literature values. Rates of SVR in our cohort were also similar to those reported in cirrhotic patients — about 50%. Due to small number of patients treated with a boceprevir-based regimen, it is difficult to compare our experience with pivotal trial experience. The side effect profiles for the two protease inhibitors were similar to the literature values except for more rectal irritation and a higher incidence and severity of anemia on telaprevir therapy in the clinical setting. While not intended to be conclusive, our study demonstrates that clinical trial data are largely compatible with the outcomes obtained in our community setting.
Keywords: HCV, triple therapy, telaprevir, boceprevir, Incivek, Victrelis, real clinical setting, community experience
Introduction
Hepatitis C virus (HCV) is the most common chronic blood borne infection in the United States.1 It is also the leading cause for liver transplants2 and liver cancer related death in the United States.3 It has been estimated that the number of people infected with chronic HCV (CHC) in the United States is 3.2 million.4 It has also been estimated that fewer than half of those living with HCV are aware that they are infected.5 In an effort to improve this discrepancy, the CDC has recently recommended screening everyone born from 1945 through 1965.6
Two new direct-acting antivirals—telaprevir (TVR) and boceprevir (BOC)—have reinvigorated treatment options for CHC. These protease inhibitors (PIs) have significantly improved outcomes for genotype 1 HCV patients when administered in combination with the previous standard of care—peginterferon (pegIFN) and ribavirin (RBV). The American Association for the Study of Liver Disease (AASLD) has therefore revised its recommendations for treatment of HCV genotype 1 patients to include triple therapy (TT).7
TVR and BOC have both been studied in large clinical trials. The ADVANCE8 and REALIZE9 trials demonstrated improved outcomes for TVR in treatment of naïve and experienced patients respectively. Similarly, the SPRINT-210 and RESPOND-211 trials demonstrated improved outcomes for BOC in treatment of naïve patients as well as prior relapsers and partial responders.
Few studies have been performed to assess the external validity of clinical trial data for TVR and BOC in a real clinical setting.12 Comparisons with real world data are important in verifying the validity and generalizability of large clinical trials.13 The purpose of this study is to compare reported data from clinical trials with outcomes in a community referral center in Honolulu, Hawai‘i.
Methods
In this retrospective study, the charts of the 59 CHC patients who were started on TT from July 1, 2011 to July 7, 2012 were reviewed. Prior to commencing therapy, patients underwent a stringent selection process. Treatment candidates were assessed for family support, current employment (to minimize potential impact of side effects on their work), insurance coverage (to aid with patient assistance program applications if required), and willingness to undergo TT. In addition, ophthalmologic, cardiac, and psychiatric consults were obtained for clearance. If psychiatric care was deemed necessary, this was managed by the consultant during and after treatment. Patients were treated with standard regimens for TVR14 and BOC15 and standard futility rules were applied.7 Adverse reactions were monitored and documented during clinical interviews. In cases where serious adverse events were encountered, treatment was withheld. Only those who reached the end point of SVR at the time of evaluation were included in the analysis of virologic outcomes, however all patients were included in the analysis of side effects.
Age, gender, ethnicity, biopsy documented cirrhosis, treatment response, and side effects were collected. Data from the ADVANCE and REALIZE trials for TVR and SPRINT-2 and RESPOND-2 trials for BOC as well as the product inserts for each agent14,15 were retrieved and used to compare with our clinical experience. Standard definitions for prior treatment categories including relapser, partial, and null responder were used, and the usual definitions for rapid virologic response (RVR), early virologic response (EVR), extended rapid virologic response (eRVR), and sustained viral response (SVR) were applied.16 Cirrhosis was defined as stage 4 fibrosis on liver biopsy.
Results
Fifty-nine CHC patients were started on TT in the study period—45 on TVR and 14 on BOC. One patient on the TVR-based regimen developed pneumonia and stopped treatment of her own accord despite having an undetectable viral load at week 4. Three patients on TVR and 1 on BOC are still on treatment or awaiting SVR. Since these patients did not meet the end point of SVR at the time of evaluation, a total of 41 patients on TVR and 13 on BOC were included in the analysis of virologic outcomes.
Table 1 displays baseline patient characteristics. In the TVR group, the median patient age was 55 years old, 71% were male and 29% were female. By self-reporting, there were 4 black patients (9%), 21 Caucasians (47%), 4 Hawaiians (11%), 13 Asians (29%), and 2 other (4%). In the BOC group, the median age was 53, 64% were male and 36% were female. There were 9 Caucasians (64%) and 5 Asians (36%). Overall, there were 13 patients with cirrhosis (22%) in our cohort—11 out of 42 in the TVR group (24%) and 2 out of 14 in the BOC group (14%).
| Telaprevir | Boceprevir | Total | |
| N | 45 | 14 | 59 |
| Median Age - yr | 55 | 53 | |
| Gender - no. (%) | |||
| Male | 32 (71) | 9 (64) | 41 (69) |
| Female | 13 (29) | 5 (36) | 18 (31) |
| Ethnicity - no. (%) | |||
| Black | 4 (9) | 0 | 4 (7) |
| Caucasian | 21 (47) | 9 (64) | 30 (51) |
| Asian§ | 13 (29) | 5 (36) | 18 (31) |
| Hawaiian | 5 (11) | 0 | 5 (8) |
| Other | 2 (4) | 0 | 2 (3) |
| Cirrhosis - no. (%) | 11 (24) | 2 (14) | 13 (22) |
| Prior treatment category - no. (%) | |||
| Treatment Naïve | 18 (45) | 7 (50) | 25 (42) |
| Prior relapsers | 14 (31) | 5 (36) | 16 (27) |
| Prior partial responders | 3 (7) | 1 (7) | 4 (7) |
| Prior null responders | 10 (22) | 1 (7) | 14 (24) |
Table 2 Key Virologic End Points and Number of Cirrhotics, According to Previous Treatment Category.*
| Telapravir | |||||
| Prior treatemtn category - no. (%) | Treatment Naïve | Prior relapsers | Prior partial responders | Prior null responders | All |
| N | 17 | 13 | 2 | 9 | 41 |
| RVR | 16 (94) | 12 (92) | 2 (100) | 7 (78) | 37 (88) |
| eRVR | 14 (82) | 12 (92) | 2 (100) | 8 (89) | 35 (85) |
| SVR | 12 (71) | 12 (92) | 1 (50) | 5 (56) | 30 (73) |
| Cirrhosis | 4 (24) | 2 (17) | 1 (50) | 1 (11) | 8 (20) |
| Boceprevir | |||||
| Prior treatemtn category - no. (%) | Treatment Naïve | Relapsers | Partial responders | Null responders | |
| N | 7 | 3 | 1 | 2 | 13 |
| RVR | 4 (57) | 2 (67) | 1 (100) | 1 (50) | 7 (54) |
| eRVR | 4 (57) | 2 (67) | 1 (100) | 0 | 7 (54) |
| SVR | 3 (43) | 2 (67) | 0 | 1 (50) | 6 (46) |
| Cirrhosis | 1 (14) | 1 (33) | 0 | 0 | 2 (5) |
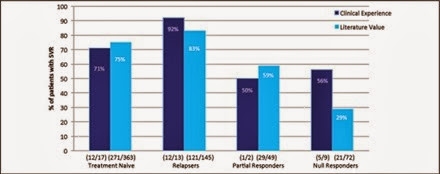
The SVR rates for TVR and BOC were 73% and 46%, respectively (Figure 2). When broken down by prior treatment category, 71% of treatment naïve, 92% of relapsers, 50% of partial responders, and 56% of null responders attained SVR in the TVR group. These results were comparable to the SVR rates observed in the ADVANCE and REALIZE trials (Figure 3). There were not enough data for BOC to meaningfully compare clinic with trial data therefore they are not shown.
Figure 2 Percentage of patients treated with protease inhibitor that achieved SVR.
Figure 3 Percentage of patients on TVR who attained SVR. Comparison by prior treatment category between our clinical experience and published literature values in the ADVANCE and REALIZE trials.
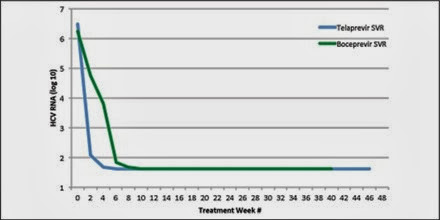
Figure 4 displays the viral kinetics for the two PI regimens for those who attained SVR. Patients treated with BOC exhibited a slower viral decline due to the standard 4-week lead-in with pegIFN/RBV without a PI. For TVR, the three-drug regimen is initiated from the outset of therapy.
Figure 4 Changes in HCV RNA levels. Shown are changes in mean log10 HCV RNA levels over the study period for patients on TVR and BOC who attained SVR.
Overall, 18 patients had virologic failure or experienced serious adverse events that required terminating treatment—11 (27%) in the TVR group and 7 (54%) in the BOC group (Table 3). In the TVR group, 1 patient was a null-responder, 4 patients had virologic breakthrough, 4 patients relapsed, and 2 termination events occurred. One patient was cirrhotic and a Jehovah's Witness and developed severe pancytopenia and renal insufficiency, that led to the only hospitalization in our cohort. The other treatment termination was due to severe thrombocytopenia in a patient with baseline thrombocytopenia and neutropenia. In the BOC group, 2 patients were null responders, 1 had virologic breakthrough, 3 relapsed, and 1 underwent treatment termination due to severe neutropenia.
Table 3 Virologic Failure and Treatment Termination.*
| Telaprevir n = 11 | Boceprevir ns = 7 | Total = 18 | |
| Null-response | 1 | 2 | 3 |
| Breakthrough | 4 | 1 | 5 |
| Termination | 2 | 1 | 3 |
| Relapse | 4 | 3 | 7 |
In our clinical experience, the SVR rates for patients with cirrhosis on TVR were compatible in each prior treatment category with those in the published literature (Figure 5). Data in the partial and null responder categories were limited and therefore not suitable for comparison.
Figure 5 Percentage of cirrhotic patients on TVR who achieved SVR. Comparison between our clinical experience and published literature values found in ADVANCE and REALIZE trials.
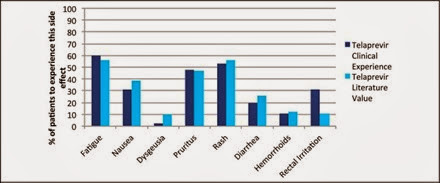
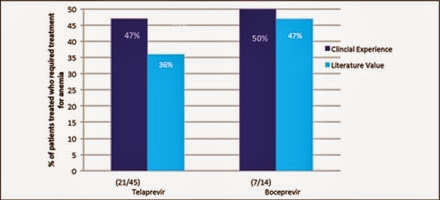
Side effects profiles in the trial data were largely similar to our clinical experience (Figure 6). Some key differences, however, were rectal irritation and anemia in the TVR group. Thirty-one percent from our cohort and 11% of clinical trial participants experienced rectal irritation. As for anemia, 47% of TVR-treated patients in our cohort had clinically significant anemia requiring treatment with either growth factors or therapeutic blood transfusions whereas the literature rate was 36% (Figure 7). Side effects for BOC including anemia were comparable.
Figure 6 TVR patient side effects in comparison to the published literature values found in the ADVANCE and REALIZE trials.
Figure 7 Percentage of patients treated with each drug that required treatment for anemia (prescribed growth factors or blood transfusions) in comparison to the published literature value for telaprevir and boceprevir.
Discussion
Virologic Outcomes
Our study demonstrates that responses to treatment and side effects profiles for TT in the community setting were largely compatible with clinical trial data. In terms of virologic end points, eRVR has been demonstrated to be a predictor of SVR. This has led to reductions in treatment duration for treatment naïve patients and prior relapsers from 48 weeks to 24 weeks—a concept known as response-guided therapy.17 For treatment naïve patients on TVR, the ILLUMINATE trial demonstrated that of the 65% of patients who attained eRVR, 92% went on to achieve SVR. In a subset analysis of the treatment naïve patients in our cohort, the eRVR rate for on TVR was 82% (Table 1); and of those 86% when on to attain SVR (data not shown). Our study was likely not powered well enough due to a small sample size to detect the extent of this predictive relationship, however the trend is similar.
In terms of SVR, the rates for TVR were compatible with published literature values in all prior treatment categories (Figure 3), which suggests the virologic outcomes obtained in the ADVANCE and REALIZE trials are valid in our clinical setting. One difference that emerged was the magnitude of the difference in SVR between null responders in our community setting versus the REALIZE trial. Generally null responders have the lowest response to therapy since they were previously refractory to pegIFN/RBV, however 56% achieved SVR in our clinical setting, the reason for which remains unclear.
Our clinical experience with BOC was limited due to the small number of patients in this group, however comparison with the published literature values in the SPRINT-2 and RESPOND-2 trials shows that the SVR rates for treatment naïve and prior partial responders were lower, while the SVR rates for prior relapsers and null-responders were near or above the literature values (data not shown).
Cirrhosis
Patients with cirrhosis represent a special subset of patients when considering CHC treatment. The FDA has approved usage of TT for cirrhotic patients, however response-guided therapy has not been recommended since historically this group has been more refractory to therapy.
The SVR rates for patients with cirrhosis in the treatment naïve and relapser categories in our clinical experience were comparable with those in the ADVANCE and REALIZE trials (Figure 5). The single cirrhotic patients in the partial and null responder categories in our cohort experienced treatment failure due to virologic relapse and breakthrough, respectively. Limited data for these groups in our cohort makes comparison with clinical trial data difficult, however the REALIZE trial also demonstrates that the stage of liver fibrosis effects outcome significantly, particularly among partial and null responders. In clinical practice, cirrhotic patients tend to develop more profound side effects, particularly bone marrow suppression necessitating dose reductions leading to decreased therapeutic efficacy. In our cohort, the two partial and null responders required RBV and pegIFN dose reductions, respectively, which may have contributed to their therapeutic failures. Recent reports also indicate high morbidity and mortality in this group of patients.18
Our clinical experience in treating cirrhotic patients with BOC again was limited. Only two patients with a fibrosis score of 4 were treated. One was treatment naïve and had a null response; the other was a relapser and achieved SVR.
Side Effects
Anemia is an important and serious side effect in the treatment of HCV. Interferon and RBV both lead to bone marrow suppression, however when combined with PIs this effect is exacerbated.19 In cirrhotic patients this effect is worsened even further since they are prone to anemia at baseline.
The number of patients treated for anemia on TVR-based regimens in our cohort was dramatically higher than the literature data.14 We postulate that this was because the combined number of patients with bridging fibrosis and cirrhosis in our cohort was higher than the ADVANCE and REALIZE trials. Cumulatively, in our TVR cohort there were 25 patients (56%) with stage 3 and 4 fibrosis compared with 20% and 50% in the ADVANCE and REALIZE trials respectively. Another possibility may have been a lower threshold for administering epoetin alpha in the clinical setting than in clinical trials. In our clinical setting, a cut off of either 10 grams or a drop of greater than 4 grams in the first 4 weeks of therapy was used as a threshold. Blood transfusions were administered when epoetin alpha did not improve hemoglobin to baseline within 1 to 2 weeks or if patients became symptomatic. Reductions in RBV doses were less aggressively applied in our clinical setting due to past experience of lower SVR rates using this strategy in the era of dual therapy.
Rectal irritation was more common in our clinical experience with TVR than in the literature. The reason for this is unclear, however it may have been due to increased provider awareness of this side effect and therefore an increased rate of self-reporting.
Other adverse side effects in our patient population were observed in a similar percentage to the literature, with the most significant side effects being fatigue, pruritus, and rash for TVR and fatigue, nausea, and dysgeusia for BOC.
In summary, this study demonstrates that outcomes in a real clinical setting were comparable to clinical trial data with some notable exceptions. The major limitation of our study was the small sample size, particularly in the BOC group, cirrhotics, and certain prior treatment categories. In addition, the influence of viral subtypes 1a and 1b and host genotype IL28B could not be analyzed in a meaningful way since, commensurate with the setting in which data were obtained, this information was not collected for all patients prior to starting therapy.
Beyond verifying compatibility between large trial and local data, this study sheds light on treatment trends in a real clinical context. Specific to the unique ethnic context in Hawai‘i, a subset analysis of Asians on TVR revealed comparable outcomes with 9 of 13 (69%) attaining SVR (data not shown). An unforeseen trend that emerged was the preponderance of patients treated with TVR. While there is no evidence to suggest that TVR or BOC offer a therapeutic advantage above the other,20,21 our experience reflects the reality that differences in utilization exist. Factors influencing PI selection include ease of administration, patient and provider discussions about side effect profiles, compliance, as well as staff training and comfort level with each regimen. Given the absence of superiority data, we propose PI preference is guided by a combination of the above factors. Another important trend that emerged was the low rate of discontinued treatment and loss to follow up. Retention is a complex issue, however, we suspect that the vast majority of patients met therapeutic endpoints in our study due to rigorous patient selection as well as close-knit relationships with community support staff ensuring individualized patient care.
Acknowledgements
The authors would like to acknowledge the contribution of our patients and their families for entrusting us with their medical care and our staff for their valuable support. Also, we would like to thank the Queen's Medical Center for supporting this research project.
Conflict of Interest
None of the authors identify any conflict of interest.
References
1. Centers of Disease Control, author. Hepatitic C Information for Health Professionals. [April 27, 2013]. http://www.cdc.gov/hepatitis/hcv.
2. Brown RS. Hepatitis C and liver transplantation. Nature. 2005 Aug 18;436(7053):973–978. [PubMed]
3. Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012 Feb 21;156(4):271–278. [PubMed]
4. Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368:1859–1861. [PubMed]
5. Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001-2008. Hepatology. 2012 Jun;55(6):1652–1661. [PubMed]
6. Centers for Disease Control, author. Recommendations for the Identification of Chronic Hepatitis C Virus Infection Among Persons Born 1945–1965. [April 27, 2013]. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6104a1.htm.
7. Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An Update on Treatment of Genotype 1 Chronic Hepatitis C Virus Infection: 2011 Practice Guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011 Oct;54(4):433–444. [PMC free article] [PubMed]
8. Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011 Jun 23;364(25):2405–2416. [PubMed]
9. Zeuzem S, Andreone P, Pol S, Lawitz E, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011 Jun 23;364(25):2417–2428. [PubMed]
10. Poordad F, McCone J, Jr, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011 Mar 31;364(13):1195–1206. [PMC free article] [PubMed]
11. Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011 Mar 31;364(13):1207–1217. [PMC free article] [PubMed]
12. Maasoumy B, Port K, Markova AA, Serrano BC, Rogalska-Taranta M, Sollik L, Mix C, Kirschner J, Manns MP, Wedemeyer H, Cornberg M. Eligibility and safety of triple therapy for hepatitis C: lessons learned from the first experience in a real world setting. PLoS One. 2013;8(2):e55285. [PMC free article] [PubMed]
13. Rothwell PM. External validity of randomised controlled trials: “To whom do the results of this trial apply?” Lancet. 2005 Jan 1–7;365(9453):82–93. [PubMed]
14. Vertex, author. Highlights of prescribing information for Telaprevir. [May 6th, 2013]. http://pi.vrtx.com/files/uspi_telaprevir.pdf.
15. Merck, author. Highlights of prescribing information for Beceprevir. [May 6th, 2013]. http://www.merck.com/product/usa/pi_circulars/v/victrelis/victrelis_pi.pdf.
16. Wedemeyer H, Jensen DM, Godofsky E, Mani N, Pawlotsky JM, Miller V. Recommendations for standardized nomenclature and definitions of viral response in trials of hepatitis C virus investigational agents. Hepatology. 2012 Dec;56(6):2398–2403. [PubMed]
17. Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, Adler M, Reesink HW, Martin M, Sankoh AJ, Adda N, Kauffman RS, George S, Wright CI, Poordad F, ILLUMINATE Study Team Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011 Sep 15;365(11):1014–1024. [PubMed]
18. Fontaine H, Hezode C, Dorival C, Larrey D, Zoulim F, De Ledinghen V, Canva V, Alric L, Bourlière M, Pol S, Poynard T, Riachi G, Bernard P, Raabe J, Gournay J, Métivier S, Pawlotsky J, Samuel D, Barthe Y, Carrat F, Bronowicki J. ANRS CO 20 CUPIC Study Group, author. SVR-12 Rate and Safety of Triple Therapy including Teleprevir or Boceprevir in 221 Cirrhotic Non-responders in the French Early Access Program (CUPIC); 48th EASL Amsterdam; April 2013; Abstract # 60.
19. Jacobson IM, Kowdley KV, Kwo PY. Anemia management in the era of triple combination therapy for chronic HCV. Gastroenterol Hepatol (NY) 2012 Sep;8(9 Suppl 6):1–16. [PMC free article] [PubMed]
20. Cooper C, Lester R, Thorlund K, Druyts E, El Khoury AC, Yaya S, Mills EJ. Direct acting antiviral therapies for hepatitis C genotype 1 infection: a multiple treatment comparison meta-analysis. QJM. 2013 Feb;106(2):153–163. [PMC free article] [PubMed]
21. Cooper CL, Druyts E, Thorlund K, Nachega JB, El Khoury AC, O'Regan C, Mills EJ. Boceprevir and telaprevir for the treatment of chronic hepatitis C infection an indirect comparison meta-analysis. Ther Clin Risk Manag. 2012;8:105–130. [PMC free article] [PubMed]



No comments:
Post a Comment