Liver International
Special Issue: Proceedings of the 7th Paris Hepatitis Conference International Conference of the Management of Patients with Viral Hepatitis, 13–14 January 2014, Paris, France. Guest Editors: Patrick Marcellin and Tarik Asselah. The publication of this supplement was supported by an unrestricted educational grant from Gilead, Janssen Therapeutics, Janssen, Bristol-Myers Squibb, Roche, Boehringer Ingelheim, Merck, AbbVie, Novartis, Idenix and Alios.
Volume 34, Issue Supplement s1, pages 69–78, February 2014
Review Article
You have free access to this content
Raymond Schinazi1,*,Philippe Halfon2, Patrick Marcellin3, Tarik Asselah3,*
Article first published online: 23 DEC 2013
DOI: 10.1111/liv.12423
© 2013 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd
Keywords: asunaprevir; daclatasvir; faldaprevir; pegylated interferon; ribavirin; simeprevir; sofosbuvir
Abstract
For HCV infection, there have been major advancements during last several years with large numbers of ongoing trials with various direct-acting antivirals (DAA) showing high potency, favourable tolerability profile, higher barrier to resistance, shortened treatment duration, all oral regimen, pan-genotypic, fewer drug interactions and reduced pill burden. By 2014, several DAAs are anticipated to complete successful phase III trials and will be commercially available. Initially, a wave of IFN-based regimen (sofosbuvir, faldaprevir and simeprevir) will be available for treatment of HCV genotype 1. In the near future, combination of antiviral agents with additive potency that lack cross-resistance with good safety profile will likely be the new recommended regimens, making HCV, the first chronic viral infection to be eradicated worldwide with a finite duration of combination DAA therapy without IFN or ribavirin. The aim of this review was to summarize the results obtained from recent DAA combination studies without IFN.
Hepatitis C virus (HCV) is a major cause of chronic liver disease, with an estimated 170 million people infected worldwide [1]. HCV, identified in 1989, is an enveloped virus with a 9.6 kb single-stranded RNA genome [2], a member of the Flaviviridae family, genus Hepacivirus. The development of new molecules called direct-acting antivirals (DAA) is ongoing [3]. The aim of this review is to summarize recent results obtained with IFN-free regimens for HCV treatment.
Viral replication cycle and targets for drug development
The HCV replication cycle begins with virion attachment to its specific receptor. The HCV RNA genome serves as a template for viral replication and as a viral messenger RNA for viral production. It is translated into a polyprotein that is cleaved by proteases followed by viral assembly. Potentially, each step of the viral cycle is a target for drug development. The knowledge of the structures of HCV protease and HCV polymerase has allowed structure-based drug design to develop inhibitors targeting these enzymes [4, 5]. Several findings suggest that HCV modulation of IFN induction and signalling attenuates the expression of IFN stimulated genes, allowing HCV to escape the antiviral actions of the host response [6, 7].
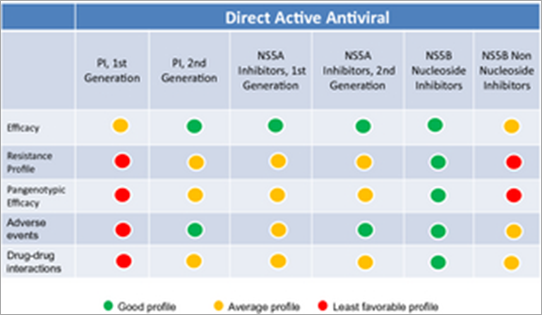
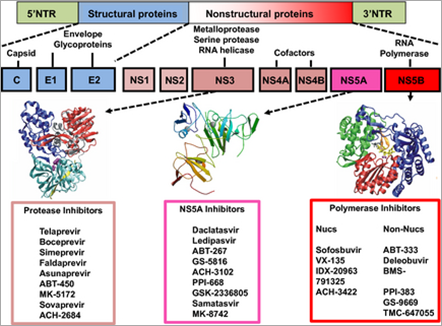
All the major HCV-induced enzymes, namely, NS2-3 and NS3-4A proteases, NS3 helicase and NS5B RNA-dependent RNA polymerase (RdRp), are essential for HCV replication and are potential drug discovery targets (Fig. 1). Therefore, DAA with different viral targets, such as NS3 protease inhibitors, nucleoside/nucleotide analogue and non-nucleoside inhibitors of the RdRp, and NS5A inhibitors are under development. General characteristics of different classes of DAA are shown in Table 1.
Figure 1. Hepatitis C virus (HCV) genome and potential drug discovery targets. The HCV RNA genome serves as a template for viral replication and as a viral messenger RNA for viral production. It is translated into a polyprotein that is cleaved by proteases. All the HCV enzymes – NS2-3 and NS3-4A proteases, NS3 helicase and NS5B RdRp – are essential for HCV replication and are therefore potential drug discovery targets.
Protease inhibitors
The NS3 serine protease is located in the N-terminal region of NS3. The NS3 serine protease domain is associated with the NS4A cofactor to cleave four specific sites.
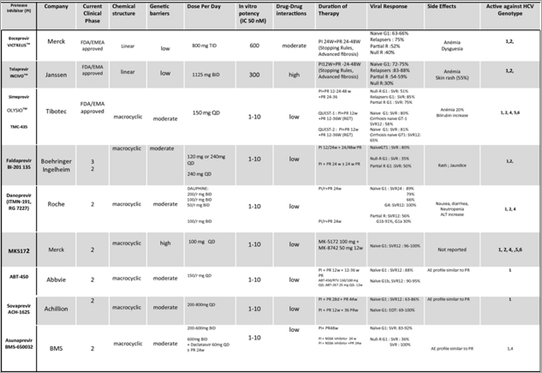
This enzyme has been extensively characterized at the biochemical level and its structure is known [4, 5]. The serine protease activity of NS3 is an attractive target for new drugs that could effectively block viral replication. The NS3/4A protease inhibitors can be divided into two chemical classes: macrocyclic inhibitors and linear tetra-peptide a-ketoamid derivatives. In 2003, a macrocyclic protease inhibitor (BILN 2061; ciluprevir) that blocks HCV replication in the replicon model was shown to be effective in humans [8-10]. Characteristics of protease inhibitors are presented in Table 2.
Although proteases inhibitors are potent, they have several potential limitations. Protease inhibitors are highly specific and as the amino acid sequence of the NS3 protease domain differs significantly between HCV genotypes, they exhibit varying activities across genotypes. For instance, telaprevir is less effective in treatment-naïve subjects infected with genotypes other than genotype 1. Furthermore, as HCV has a high mutation replication rate, with a lack of proofreading, resistance is an issue for this class of drugs.
The genetic barrier to resistance is defined as the number of amino acid substitutions required to confer full resistance to a drug. Usually, DAA with a low genetic barrier to resistance require only one or two amino acid substitutions for high resistance. DAA with a high barrier to resistance usually require three or more amino acid substitutions in the same region to confer loss of activity.
The genetic barrier to protease inhibitors is usually low and resistance differs significantly between HCV genotypes. Viral resistance to telaprevir occurred much more frequently in genotype 1a compared with genotype 1b. This is believed to be the result of nucleotide differences at position 155 in HCV subtype 1a (AGA, encodes R) vs. 1b (CGA, also encodes R). The mutation most frequently associated with resistance to telaprevir was R155K; changing R to K at position 155 requires only one nucleotide change in HCV subtype 1a and two nucleotide changes in subtype 1b isolates [11] making GT1a more susceptible to emergence of resistance. As illustrated with the R155K mutation, which reduces replication capacity in the replicon model [12], resistance mutations frequently impair viral fitness. However, under antiviral pressure, during continued therapy, second site mutations are selected that restore fitness, explaining why the R155K primary mutation is frequently found in association with V36M in genotype 1a viruses. Therefore, it is recommended to immediately discontinue treatment in subjects with viral breakthrough and good adherence to therapy.
The main weaknesses of the first-generation PIs are their low genetic barrier to resistance and the fact that their effectiveness is limited to GT-1 patients. Second-wave PIs have a higher barrier to resistance, better activity against multiple genotypes except GT-3, more convenient dosing schedules and improved safety and tolerance [13-18]. Second-generation PIs are compounds that are broadly active against all genotypes and against viral isolates that carry resistance mutations for first-generation PIs. In combination with PR, the new PIs appear to achieve greater SVR rates than the first-generation PIs. These new treatments allow for more convenient administration schedules (one or two administrations per day); this could result in improved pharmacokinetics and better patient compliance. Besides, the safety profile seems to be good. The pan-genotypic activity of these new treatments provides new therapeutic options for a greater number of patients, in particular for those infected with GT-4.
Table 2 provides an overview of the efficacy and tolerance of the second-wave PIs that are currently developed. Few data are available concerning second-wave PIs for cirrhotic patients. In a phase IIb study, 83 GT-1 treatment-experienced cirrhotic patients were treated with simeprevir 100 or 150 mg QD and PR for 12, 24 or 48 weeks followed by PR alone up to week 48. The SVR rates were 73% for previous relapsers, 82% for partial responders and 31% for null responders (for those treated with 150 mg QD); in all cases, the SVR rates were higher than in the PR arms.
In the pivotal Phase 3 trials, C208, C216 and HPC3007, simeprevir in combination with PR was demonstrated to be superior to placebo (in combination with PR) in achieving an SVR in both HCV treatment-naïve subjects and relapsers [13, 14]. In the subgroup of subjects with the Q80K baseline polymorphism, a substantial impact on the efficacy of simeprevir was observed.
Polymerase inhibitors
Polymerase inhibitors interfere with viral replication by binding to the NS5B RNA-dependent RNA polymerase. NS5B RNA polymerase inhibitors can be divided into two different types – nucleoside inhibitors (NI) and non-nucleoside inhibitors (NNI). NI mimic the natural substrates of the polymerase and are incorporated into the RNA chain causing direct chain termination [19, 20]. NI are compounds that require conversion to an active triphosphate form. As the active site of NS5B is highly conserved, NI are generally pan-genotypic (effective against all the different genotypes). However, single amino acid substitutions in every position of the active site may result in loss of function of the NI, but resistance to nucleoside analogue inhibitors is typically very low in humans as this virus has reduced fitness.
In contrast, NNI bind to several discrete sites outside of the HCV polymerase active centre, which results in conformational protein change before the elongation complex is formed [19, 20]. NS5B is structurally organized in a characteristic ‘right-hand motif’ containing finger, palm and thumb domains, and offers at least four NNI-binding sites, namely, benzimidazole (thumb 1)-binding, thiophene (thumb 2)-binding, benzothiadiazine (palm1)-binding and benzofuran-(palm 2)-binding sites.
Resistance is more frequent with NNI compared with NI. However, mutations at NNI-binding sites do not necessarily lead to impaired function of the enzyme. Characteristics of polymerase inhibitors are presented in Table 3.
NS5A inhibitors
The NS5A is a membrane-associated phosphoprotein present in basally phosphorylated (p56) and hyperphosphorylated (p58) forms [20-22]. It was previously reported that only p58-defective mutants could be complemented in trans, and NS5A is involved in HCV virion production, suggesting that different forms of NS5A exert multiple functions at various stages of the viral life cycle [21, 22]. The N terminus of NS5A (domain I) has been crystallized in alternative dimeric forms and contains both zinc- and RNA-binding domains, properties that have been demonstrated in vitro. NS5A has been shown to interact with a number of host proteins and plays a role in interferon resistancein vivo [20, 21]. Daclastavir is active at picomolar concentrations in vitro in HCV replicons expressing a broad range of HCV genotypes and acts in an additive to synergistic fashion with interferon and other DAAs [20-22]. The resistance profile of daclastavir reveals inhibitor sensitivity maps to the N terminus of domain 1 of NS5A [21]. It has been demonstrated that NS5A inhibitors could block hyperphosphorylation of NS5A, which is believed to play an essential role in the viral replication cycle.
Interferon-free combination trials
Several IFN-free combination trials are ongoing with different DAAs that target multiple viral sites: NS3/4a protease inhibitors, NS5B polymerase inhibitors (NI and NNI) and NS5A inhibitors. There have been major advancements in the last several years with large numbers of trials with various DAA showing increased SVR rates, favourable tolerability and shortened treatment duration with all oral regimens. The priorities for future combination are listed in Table 3. Fortunately, there will be opportunities to reduce cross-resistance[23]. Among unmet need, genotype 4-infected subjects need to be considered. Approximately 20% among the 170 millions of HCV-infected subjects worldwide are genotype 4 (approximately 34 millions). The standard treatment for HCV GT4 is PEG-IFN plus ribavirin for 48 weeks. Naive GT4 IL28B non-CC subjects have SVR rates lower than 50% with the standard PEG-IFN plus ribavirin for 48 weeks [24]. Furthermore, GT4 previous relapsers or non-responders have very low chance of being cured with the same regimen.
HCV drug development is shorter than, for example, HIV drug development because of short treatment duration, the option of open-label studies without the need of a control arm and also the primary end point for efficacy is SVR12 (12 weeks post-treatment follow-up), which is as relevant as 24 weeks to determine the SVR [25]. At present, several advanced studies of DAA combinations are ongoing, especially in more difficult-to-cure infected individuals.
IFN-free regimen for genotype 1-naïve and -experienced subjects
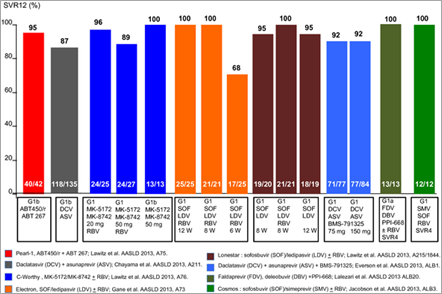
Results of IFN-free DAA regimens in treatment-naive GT1 individuals are presented in Figure 2 and for treatment-experienced GT1 in Figure 3.
Faldaprevir with or without RBV (Boehringer-Ingelheim)
SOUND-C2 is an open-label, randomized, Phase IIb study that enrolled 362 treatment-naïve HCV genotype-1 subjects into one of five treatment arms. The study evaluated the safety and efficacy of faldaprevir (protease inhibitor) and deleobuvir (polymerase inhibitor), with and without RBV [26, 27]. Final results from this study showed that up to 85% of HCV individuals infected with genotype-1b (GT-1b) achieved SVR. The optimal regimen was 28 weeks of faldaprevir (QD) and deleobuvir (BID). This study, which was the largest interferon-free trial of its kind to be conducted to date, included persons with cirrhosis. SVR was achieved in 70% overall subjects, compared with 85% seen in the prevalent GT-1b subject subgroup. Nine per cent of the total population had cirrhosis and this subgroup achieved SVR rates of up to 67% [26].
The most common adverse events (AEs) in SOUND-C2 were mild skin changes (itchy skin, rash or photosensitivity) or gastrointestinal disorders and transient indirect hyperbilirubinemia which sometimes presented as jaundice. Thirty six per cent of subjects experienced an AE, of which 12% were considered severe and 8% led to discontinuation of treatment. IFN-free phase III studies are ongoing.
Furthermore, faldaprevir plus deleobuvir plus PPI-668 (NS5A inhibitor) with or without ribavirin in persons with genotype 1a infection was studied [28]. Thirty-seven individuals with GT1a (without cirrhosis) were included. At week 4, HCV RNA was undetectable (<25 IU/ml) for 97% of subjects (35/36). SVR4 was available for 13 persons, and all had undetectable HCV RNA.
Aviator study: ABT-450/r, ABT-267, ABT-333 (Abbvie) with or without RBV
The Aviator phase 2b study assesses the safety and efficacy of ABT-450/r (dosed 100/100 mg to 200/100 mg QD), ABT-267 (25 mg QD), ABT-333 (400 mg BID) and RBV (weight based dosing) in non-cirrhotic treatment-naïve subjects and in prior PEG-IFN/RBV null responders for 8, 12 or 24 weeks [29]. ABT-450 is a ritonavir-boosted protease inhibitor [30]; ABT-267 is an NS5A inhibitor and ABT-333 is an NS5B polymerase NNI. Enrolment was open to GT1-infected individuals regardless of IL28B host genotype. SVR12 in treatment-naïve genotype 1 (GT1) subjects was 97.5% (77 of 79) and 93.3% (42 of 45) in GT1 null responder subjects. In GT1a subjects, SVR12 was achieved in 96% (52 of 54) of treatment-naïve subjects and 89% (25 of 28) of null responder subjects. In GT1b subjects, SVR12 was achieved in 100% of treatment-naïve (25 of 25) and null responder subjects (17 of 17). In addition, a separate Phase 2a, open-label study was conducted in treatment-naive and partial/null responders administered treatment for 12 weeks. A total of 19 subjects previously untreated subjects were enrolled in group 1, 14 previously untreated persons in group 2 and a total of 17 subjects with a null or partial response to previous therapy in group 3. Results from the 12-week triple-DAA regimen without RBV in treatment-naïve subjects showed that SVR12 was achieved in all individuals who completed treatment (95%) in group 1, 93% in group 2 and 47% in group 3.
The treatment was well tolerated. There was one treatment discontinuation in group 1 because of elevated levels of aspartate aminotransferase and alanine aminotransferase at week 2. No serious adverse event or death occurred in this study. The most common AEs were fatigue (47, 43 and 35%), nausea (21, 21 and 24%) and headache (26, 14 and 18%) for groups 1, 2, and 3 respectively.
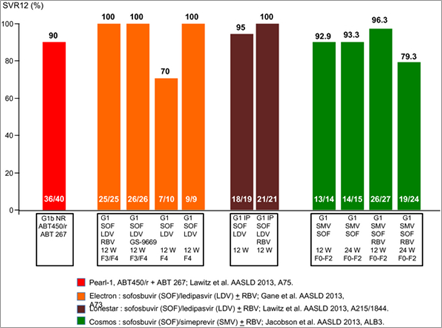
Furthermore, ABT-450/r plus ABT-267 regimen was studied in genotype 1b-naive subjects (n = 42) and null responders (n = 40); all without cirrhosis [31] (PEARL I). SVR of 95.2% for treatment-naive subjects, and 90% for null responders was reported. The triple-DAA combination is currently being studied in Phase III clinical trials.
Sofosbuvir, GS-5885 and ribavirin (Gilead) (Electron study)
Interim data from the ongoing Phase 2 Electron study examining a 12-week course of therapy with the NS5B nucleotide inhibitor sofosbuvir, the NS5A inhibitor GS-5885 and ribavirin in subjects with genotype 1 chronic hepatitis C virus (HCV) infection were reported[32]. Among treatment-naïve individuals receiving this combination, 100% (n = 25/25) remained HCV RNA undetectable 4 weeks after completing therapy (SVR4). Among the nine genotype 1 previous null responders who were treated with sofosbuvir, GS-5885 and ribavirin for 12 weeks, three of the nine subjects have reached the 4-week post-treatment time point and all three remain HCV-negative. Both sofosbuvir in combination with RBV or GS-5885 plus RBV were well tolerated in this study. The most common AEs were headache, fatigue, upper respiratory tract infection and nausea. The most common clinically significant grade 3/4 laboratory abnormality was a haemoglobin reduction.
Phase 3 trial (ION-I) evaluating a fixed-dose combination of sofosbuvir and GS-5885 in treatment-naïve genotype 1 subjects are ongoing. This four-arm study is evaluating the fixed-dose combination with or without ribavirin for 12- and 24-week durations in 800 subjects, 20% of whom have evidence of cirrhosis.
Daclastavir (Bristol Myer Squibb) plus Sofosbuvir (Gilead) with or without RBV
This phase II trial was designed to test the combination of daclatasvir (NS5A inhibitor) and sofosbuvir in HCV GT1, 2 and 3, with or without RBV, for 12 or 24 weeks of therapy, and with or without a week-long run-in period with sofosbuvir [33].
A total of 44 subjects with the viral genotypes 2 and 3 were enrolled in three arms – one with a 7-day sofosbuvir run-in period followed by 23 weeks of the two together, one with the combination for 24 weeks and one with the combination plus ribavirin for 24 weeks. Eighty-eight per cent of subjects in the first group reached an SVR12, compared with 100% in the second group and 86% in the third group.
In genotype 1, the trial had 3 arms, with a total of 44 subjects with the same regimens as in the genotype 2/3 subjects. They also tested the combination with and without RBV for 12 weeks in a total of 82 subjects. All subjects receiving the first three regimens achieved an SVR12 and, all but one remained undetectable at SVR24. It was reported that out of the 82 subjects in the 12-week arms, 68 had reached 12 weeks post-treatment and all had SVR12.
Daclatasvir, asunaprevir and BMS-791325 (BMS)
Daclatasvir is the first NS5A replication complex inhibitor to be investigated in HCV clinical trials and is currently in Phase III development. Asunaprevir is an NS3 protease inhibitor in Phase III development with daclatasvir. BMS-791325 is a NS5B polymerase NNI, currently in Phase II development for HCV as a component of daclatasvir-based treatment regimens. A Phase II study evaluated the above three different classes of DAAs – daclastavir, asunaprevir and BMS-791325 administered for 12 or 24 weeks in treatment-naïve persons with genotype 1 chronic HCV infection [34]. In the 24-week group, 94% achieved SVR4 and in the 12-week treatment group, SVR12 was achieved in 94% of persons. One hundred sixty-six naive GT1 subjects were treated (GT1a 82%; cirrhosis n = 15). SVR12 was 91% for GT1a and 94% for GT1b. Phase III trials with three DAA fixed-dose combination (BID) are anticipated.
Daclatasvir and asunaprevir in genotype 1b prior null responders
Previous data on daclastavir (NS5A inhibitor) and asunaprevir (protease inhibitor) have reported exciting results in genotype 1b null responders [35]. A phase III trial of daclatasvir plus asunaprevir was undertaken that evaluated either IFN ineligible naive/intolerant (n = 135) and non-responders to prior IFN-based therapy (n = 87) in Japanese subjects with genotype 1b infection. The study reported SVR24 rate of 87% in IFN ineligible/intolerant individuals and 81% in non-responders [36].
MK-5172 (QD) plus MK-8742 (QD) (NS5A inhibitor) with or without ribavirin (C-WORTHY Study) (MSD)
This is a Phase 2 study (n = 65) evaluating the combination of once-daily MK-5172 (protease inhibitor) plus MK-8742 (NS5A inhibitor) with or without ribavirin, administered for 12 weeks in genotype 1a- and 1b-naive subjects [37]. Remarkably, the two arms achieved an SVR12 of 100%: MK-8742 dose of 20 mg/day with ribavirin (21/21) and MK-8742 dose of 50 mg/day without ribavirin (12/12), both in combination with 100 mg/day MK-5172.
Simeprevir plus sofosbuvir with or without ribavirin in GT1-naive subjects and prior null responders (COSMOS study)
COSMOS is a Phase 2a, randomized, open-label study that evaluated once-daily combination of protease inhibitor, simeprevir plus sofosbuvir with or without ribavirin for 12 or 24 weeks in GT1-naive subjects (cirrhotic and non-cirrhotic) and prior null responders [38]. Cohort 1 (n = 80) randomized prior null responders persons with METAVIR scores F0-F2 and Cohort 2 (n = 87) evaluated prior null responder and treatment-naïve GT1 individuals with METAVIR scores F3-F4.
In cohort 1, prior null responders with Metavir F0-F2, SVR8 was 93% (without ribavirin) and 96% (with ribavirin). Viral relapse was observed in three subjects, all in GT1a with Q80K polymorphism mutation. In cohort 2, SVR4 results from the 12-week groups was 96% (with RBV) and 100% (without RBV). SVR4 in cirrhotics was 94% (17/18).
IFN-free regimen for genotype non-1 subjects
Data from several phase III studies of sofosbuvir for genotype non-1 subjects are available [39-41].
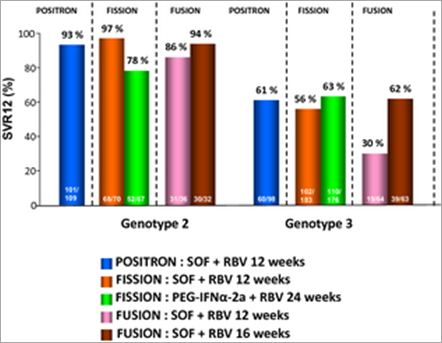
The FISSION trial was a randomized, open-label, active-controlled, phase III study of sofosbuvir plus RBV in naïve subjects with GT2 or GT3 HCV infection; subjects with the two genotypes were enrolled in approximately 1:3 ratio [39]. Subjects were randomly assigned in a 1:1 ratio to receive either 12 weeks of sofosbuvir plus RBV or 24 weeks of PEG-IFN/RBV. The doses of sofosbuvir and RBV were the same as those administered in the Neutrino trial. The dose of RBV for subjects in the PEG-IFN/RBV group was 800 mg daily. Sofosbuvir–RBV was shown to be non-inferior to PEG-IFN/RBV. At 12 weeks, the rates of SVR for subjects receiving 12 weeks of sofosbuvir/RBV and those receiving 24 weeks of PEG-IFN/RBV were each 67%. A SVR occurred in 97% of subjects with GT2 and in 56% of those with GT3 in the group receiving sofosbuvir/RBV, as compared with response rates of 78 and 63%, respectively, in the group receiving PEG-IFN/RBV. Among subjects with cirrhosis at baseline, 47% of those receiving sofosbuvir/RBV had a SVR, as compared with 38% of those receiving PEG-IFN/RBV.
The POSITRON trial was a blinded, placebo−controlled phase III study that compared 12 weeks of treatment with sofosbuvir and RBV with matching placebo in GT2 and GT3 HCV-infected subjects who had previously discontinued IFN-therapy because of unacceptable adverse events, who had a concurrent medical condition precluding therapy with an IFN−containing regimen, or who had decided against treatment with an IFN−containing regimen [34]. The most common reasons that IFN treatment was not an option were clinically significant psychiatric disorders (in 57% of subjects) and autoimmune disorders (in 19%).
The rate of SVR at 12 weeks after treatment was 78% among subjects receiving sofosbuvir/RBV compared with 0% among those receiving placebo (P < 0.001). Among subjects who received sofosbuvir/RBV, 93% of subjects with GT2 HCV infection had an SVR compared with 61% with GT3 HCV infection. Likewise, 81% of subjects without cirrhosis (92% of subjects with GT2 HCV infection and 68% of those with GT3 HCV infection) had a SVR as compared with 61% of subjects with cirrhosis (94% of subjects with GT2 HCV infection and 21% of those with GT3 HCV infection).
The FUSION study was a blinded, active−controlled phase III study involving GT2 and GT3 HCV-infected subjects who had no response to prior treatment with an IFN−containing regimen [40]. Approximately 75% of the previously treated subjects enrolled had either virological breakthrough during the prior treatment or virological relapse afterwards; the remainder did not have a response. The rates of SVR achieved were superior to the historical control rate of 25%, with rates of 50% in the 12-week group and 73% in the 16-week group (P < 0.001 for each comparison). Rates of SVR between the groups showed that subjects receiving 16 weeks of treatment had a significantly higher rate of SVR than subjects receiving 12 weeks of treatment (P < 0.001). The rates of SVR among subjects with GT2 HCV infection who received 12 weeks of treatment and those who received 16 weeks of treatment were 86 and 94%, respectively, compared with 30 and 62% for 12 and 16 weeks of treatment, respectively, among subjects with GT3 HCV infection.
Cirrhosis was associated with a decreased rate of SVR, particularly among subjects with GT3 HCV infection who received 12 weeks of treatment. Among subjects with cirrhosis who received 12 weeks of treatment, the rate of SVR was 31% (60% with GT2 HCV infection and 19% with GT3 HCV infection) as compared with 61% among subjects without cirrhosis (96% with GT2 HCV infection and 37% with GT3 HCV infection). Among subjects with cirrhosis who received 16 weeks of treatment, the rate of SVR was 66% (78% with GT2 HCV infection and 61% with GT3 HCV infection) as compared with 76% among subjects without cirrhosis (100% with GT2 HCV infection and 63% with GT3 HCV infection). Results of these trials are summarized in Figure 4.
Figure 4. IFN-free trials for non-genotype 1 subjects
Conclusion
The standard of care for treatment of HCV GT1 improved with the introduction of telaprevir and boceprevir in 2011, which is used in combination with peg-IFN and RBV triple therapy. Triple therapy has improved SVR rates and treatment durations for many individuals with GT1 HCV infection. However, there has been a marked paradigm shift in the management of HCV infection as a result of the promising outcomes from recent studies with DAA combinations reporting increased SVR, low or no resistance and a good safety profile. There is a realistic hope for an all oral regimen against HCV in the near future, as several compounds with different mechanisms of action with synergistic interactions and pan-genotypic activity are in advanced drug development.
However, limitations to HCV treatment still exist particularly with comorbid conditions and in difficult-to-cure persons with advanced liver disease including those with decompensated cirrhosis. These individuals on newer therapy still have additional treatment-limiting adverse events and drug interactions. Furthermore, issues such as resistance and treatment failures, especially in the previously treated population, will need to be overcome with new drugs and combinations. Additional guidance may be obtained by genetic testing, but availability of alternative regimens for therapy is still needed for HCV. A pangenotypic once-daily or weekly regimens that will treat all populations with an SVR12 of greater than 95% is needed that can even be used safely in children and to prevent mother-to-child HCV transmission would be the ultimate goal. However, much progress has been made since HCV was first discovered in 1989. Based on the recent encouraging results from the widely studied IFN-free regimens in non-GT1 infection, it is possible that in the future, HCV may be the first chronic viral infection to be eradicated worldwide with one or more antiviral drug. The concept of treatment as prevention and a cure is gaining traction and will need to be applied globally to eradicate this virus from the face of the earth as we succeeded with smallpox in 1977.
Perspectives and therapeutic strategies
Simple strategies with complexes combination based or not based using back-bone of Nucleoside inhibitors are ongoing development regarding the availability of the new DAA:
In genotype 1 patients
- Using nucleoside analogues the combination with PR+sofosbuvir have to be in balance with the IFN-free based on NI+NS5A±RBV or NI+PI±RBV
- Without using nucleoside analogues: the combination with PR+PI have to be in balance with the IFN-free based on PI+NS5A±RBV or PI±NNI+RBV or PI±NNI+NS5A+RBV
In genotype 2 and 3 patients
- i. Genotype 2: the combination of sofosbuvir plus RBV for 12 weeks that leads to SVR higher than 90% might be the next standard of care
- ii. Genotype 3
Naïve: the combination of sofosbuvir plus RBV for 24 weeks that leads to SVR around 80%, or other DAAs in the near future, may be proposed
Treatment Experienced:
- iii. In non-cirrhotic patients, the combination of SOF+RBV for 24 weeks-treatment lead to a 85% SVR
In cirrhotic patients, the combination of PR+RBV+SOF for 12 weeks of treatment leading to a 83% SVR has to be in balance with the combination of SOF+RBV for 24 weeks of treatment, which leads to a 60% SVR. In genotype 4 patients, there will be several possibilities: PEG-IFN plus RBV, combination of SOF + RBV for 16–24 weeks, triple therapy with SOF plus PEG-IFN/RBV, or Simeprevir plus PEG-IFN/RBV for 12–24 weeks. Future IFN-free regimen might be available for HCV genotype 4-infected patients.
Acknowledgments
This work was supported in part by CFAR NIH grant 2P30AI-050409 (to RFS) and by the Department of Veterans Affairs (to RFS). We thank Judy Mathew and Steve Coats for proofing this manuscript. Dr. Schinazi is the founder and a major shareholder of RFS Pharma, LLC.
Conflicts of interest: Tarik Asselah is a speaker and investigator for BMS, Boehringer-Ingelheim, Tibotec, Janssen, Gilead, Roche and MSD. Patrick Marcellin is a speaker and investigator for BMS, Boehringer-Ingelheim, Tibotec, Janssen, Gilead, Roche and MSD. Raymond Schinazi is the founder and major shareholder of RFS Pharma, LLC. Philippe Halfon is a speaker for Roche, Merck, Janssen and shareholder of Alphabio and Genoscience.

No comments:
Post a Comment