Journal of Clinical & Experimental Hepatology
Volume 3, Issue 4 , Pages 337-346, December 2013
Dronacharya Routh, Sudeep Naidu, Sanjay Sharma, Priya Ranjan, Rajesh Godara
Received 18 November 2013; accepted 18 November 2013. published online 25 November 2013.
During the last couple of decades, with standardization and progress in surgical techniques, immunosuppression and post liver transplantation patient care, the outcome of liver transplantation has been optimized. However, the principal limitation of transplantation remains access to an allograft. The number of patients who could derive benefit from liver transplantation markedly exceeds the number of available deceased donors. The large gap between the growing list of patients waiting for liver transplantation and the scarcity of donor organs has fueled efforts to maximize existing donor pool and identify new avenues. This article reviews the changing pattern of donor for liver transplantation using grafts from extended criteria donors (elderly donors, steatotic donors, donors with malignancies, donors with viral hepatitis), donation after cardiac death, use of partial grafts (split liver grafts) and other suboptimal donors (hypernatremia, infections, hypotension and inotropic support).
Keywords: extended criteria donor, liver transplantation, donor pool
Abbreviations: LT, liver transplantation, ECD, extended criteria donor, DCD, donation after cardiac death, PNF, primary nonfunction, DGF,delayed graft function, LDLT, living donor liver transplantation, SLT, split liver transplantation, CIT, cold ischemia time, MELD, Model for End-Stage Liver Disease, SRTR, Scientific Registry of Transplant Recipients, SOFT, survival outcomes following liver transplantation, mTOR,mammalian target of rapamycin inhibitors, HIV, human immunodeficiency virus, HBV, hepatitis B virus, HCV, hepatitis C virus, HBIg, hepatitis B immune globulin, HTLV, human T-lymphotropic virus, NRP, normothermic regional perfusion, ECMO, extra corporeal membrane oxygenation
Liver transplantation (LT) has been the only proven treatment for patients with end-stage liver disease or hepatocellular carcinoma in cirrhotic patients. However, with increasing success in the outcome of LT there has been further expansion of indications. Thus with the widening of indications the demand for organs has been increasing steadily and exceeds the number of available deceased donors. Gradually, live donation and technically modified grafts were introduced and accepted as a means of enhancing the donor organ pool. Unlike India and Asian countries most transplant centres in the world still depend on the deceased donor pool as opposed to live donation.
A major challenge for the transplant community is to develop strategies to close the gap between the number of patients in need of a transplant and the number of available organs. One of the main strategies to address this discrepancy is expansion of the Deceased Donor pool utilizing extended criteria donor (ECD) and donation after cardiac death (DCD) donors.1, 2, 3 This can be done by using organs that were previously thought to be associated with a high risk of primary nonfunction (PNF) or delayed graft function (DGF), the so-called ECD or marginal livers3, 4 (i.e., donors with steatosis, with malignancies, with viral infections, older or elderly donors, DCD, etc). These livers considered unacceptable for transplantation in the past, are now being considered for transplantation. Another way to expand the donor pool is through advances in medical practice, particularly surgical techniques including living donor liver transplantation (LDLT) and split liver transplantation (SLT). Although the ECD organs may not be optimal, the high death rate on the waiting lists has forced transplant surgeons to make a stark choice between dying without a liver and proceeding with a liver that was perhaps not ideal.1, 2, 3, 4 It is also known that the marginal grafts exhibit poor tolerance to ischemia/reperfusion (I/R) injury, which is an important cause of liver damage occurring during surgical procedures including hepatic resections and LT.1, 3
Change in donor characteristics: natural history
There has been a paradigm shift in the organ donation trends over the last couple of decades. During the era of expansion of automobile industry with minimal road safety measures, the majority of deceased donors consisted young victims of road traffic accidents. These donors were less likely to have steatosis and in the absence of significant comorbidity, their liver function was more likely to be good at the time of donation. However, with improved road safety measures there has been a decrease in the number and severity of traffic accidents and fewer trauma patients as potential donors. The current socioeconomic trend has shown that donors are more likely to be obese than before and with increase in the average life expectancy of people more potential donors are likely to be in advancing age group and may have active or previous history of treated malignancy.5 So if we go by the original yardstick of an “ideal” donor, then these factors will probably make a donor “extended criteria donor or marginal donor”.6, 7
Ideal donor: definition
An ideal or reference donor was defined according to the following criteria: age below 40 years, trauma as the cause of death, donation after brain death, hemodynamic stability at the time of procurement, no steatosis or any other underlying chronic liver lesion, and no transmissible disease.8, 9 A reference donor implies a very low risk of initial poor function or early allograft failure leading to death or requiring re-transplantation. An ideal allograft is different from an ideal donor. The ideal allograft category may be influenced by variables that are introduced following procurement, such as the prolonged ischemia time (CIT), or technical variants, such as those occurring with allograft reduction (e.g., split liver allograft). These variables should not be included in the definition of ECD because the aim is to assess risk at procurement.
Extended criteria donor: definition
An extended criteria donor implies higher risk in comparison with a reference donor. The risk may manifest as increased incidence of poor allograft function, allograft failure, or transmission of a donor-derived disease. Adhering to strict donor selection criteria is safe but unlikely to help reduce transplant wait list mortality. Exploring the possibility of using grafts from extended criteria donors is advantageous in many ways. Provided the decision-making is performed with a “utilitarian” approach, sicker patients are likely to face less competition on the wait list whenever a “conventional” or good quality graft becomes available. In turn, this may help reduce the transplant wait list mortality benefiting the largest number of recipients.10
Donor characteristics in liver transplant: how important is it?
With increasing gap between donor organ availability and patients in need of transplantation, the use of marginal high risk or ECD organs has increased.11 Though priority in liver allocation is based on the Model for End-Stage Liver Disease (MELD) score, donor-recipient matching occurs at the time of organ procurement and transplantation, and substantial selection is involved in accepting an organ.12 The identification of donor-related factors that portend poor posttransplant outcomes and analysis that can guide the use of organs according to donor characteristics have become increasingly important,13especially because donor characteristics and medical management vary by region and organ procurement organization and may affect posttransplant outcomes.8The most important donor factor is age, which has repeatedly been shown to be a significant predictor of allograft failure and posttransplant death.8, 13, 14, 15, 16, 17 This is especially true for patients undergoing transplantation for HCV; outcomes are significantly worse for patients with HCV who receive livers from older donors. Less is understood about the effects of older donor allografts, especially with respect to long-term outcomes, in non-HCV recipients. The type of donor is also important; the use of DCD livers is associated with an increased risk of posttransplant allograft failure.18, 19 The MELD score is an excellent predictor of wait list mortality but a suboptimal predictor of posttransplant allograft and patient survival because of donor, recipient, and transplant characteristics and unpredictable posttransplant events (e.g., patient compliance, allograft primary nonfunction, and hepatic artery thrombosis). Objective parameters that quantify the risk associated with donor organs are actively being sought. Several mathematical models have been proposed to identify predictors of allograft and patient survival after liver transplantation.
Feng, et al,8 analyzed 20,000 transplants from the Scientific Registry of Transplant Recipients (SRTR) database and developed a DRI, which is calculated from seven donor and two transplant variables that were found to be independently associated with an increased risk of graft failure. These included donor >40 years, donor height, donation after cardiac death, split/partial grafts, cerebrovascular accident or other cause of death (except trauma, stroke, or anoxia), cold ischemia time, and organ sharing outside the local donor service area. Although a conclusive statement on the impact of graft steatosis could not be made due to incomplete data in the registry, the analysis of Feng, et al,8 highlights the relevant donor risk factors and supports a clear correlation between organ quality and post-transplantation outcome.
Similarly, Rana et al20 identified 13 recipient factors, 4 donor factors, and 2 operative factors (warm and cold ischemia times) as significant predictors of recipient mortality 3 months after transplantation, using MELD era data and including retransplants. Using 18 risk factors, (excluding the warm ischemia time), the survival outcomes following liver transplantation (SOFT) score successfully predicted 3-month recipient survival. The SOFT score included the MELD score at the time of transplantation (categorized as >30 or <30). In their analysis of predicting 3-month mortality after liver transplantation, the concordance statistic was 0.63 for the MELD score and 0.70 for the SOFT score. In comparison, the MELD score c statistic was greater than 0.85 for predicting wait list mortality.21 Donor race was not a significant predictor in this study. Concerns similar to those outlined previously and complex statistical modeling limit its widespread application. Furthermore, longer time periods are needed to judge successful transplants; 3-month mortality estimates may be highly influenced by perioperative factors, which may be indirectly related to transplant center characteristics.
Donor physiology and brain death prior to procurement
Brain death is associated with a number of circulatory, metabolic, and hormonal changes eventually leading to somatic death and circulatory changes are the leading cause of organ dysfunction.22, 23 There are no guidelines on the care of donors with respect to optimizing liver allograft function prior to procurement. Donor homeostasis has been defined by a mean arterial pressure between 65 and 100 mm Hg, urine output between 1 and 1.5 mL/kg/h, hemoglobin between 7 and 9 g/dL, normal arterial blood lactate, partial pressure of arterial oxygen over 80 mm Hg, temperature between 35.5 °C and 38 °C, and serum sodium below 150 mmol/L.24 Accumulated data, both in animal models and in humans, have demonstrated dysfunction of the hypothalamic-pituitary-adrenal axis during brain death that leads to a decrease in circulating thyroid hormone and corticosteroids.25 However, no clear evidence exists indicating that exogenous hormone therapy (thyroid hormones and/or corticosteroids) improves transplant outcomes.26, 27 Additional areas of future research include the potential usefulness of nutritional support, glycine, and N-acetyl cysteine.28, 29, 30
Factors affecting the outcome of use of extended criteria donor
Cold Ischemia Time
Prolonged CIT is an independent risk factor for the development of delayed graft function and primary nonfunction.31Recipient survival was shown to be adversely affected by CIT over 12 h in a European survey and over 10 h in a US survey.32,33 The European Liver Transplant Registry survey showed that 5-year recipient survival was 57% with CIT over 15 h versus 64% with CIT between 12 and 15 h and 67% with CIT below 12 h.34 Liver grafts from elderly donors and/or donors with steatosis are even more affected by prolonged CIT and preservation injury. In this group, optimal liver function can be best achieved when CITs are kept less than 8 h.35 These results emphasize the need to shorten CIT as much as possible in the case of extended criteria donors.
Preservative Solutions
The development of the University of Wisconsin (UW) preservation solution has dramatically improved the quality of preserved allografts.36, 37, 38, 39 They have been designed to reduce cellular injury during cold ischemia and minimize reperfusion injury. UW has been used throughout the world for more than 20 years but is now challenged by 3 other solutions—Celsior, histidine tryptophan ketoglutarate, and IGL-1—which are less expensive and potentially superior for organ preservation.40, 41, 42 No difference in short-term or long-term outcomes has been observed for each of these 3 solutions in comparison with UW.40, 41, 42, 43 However, the study populations in the trials that have been reported so far are relatively small and nonselect groups of donors. The lower viscosity of histidine tryptophan ketoglutarate and Celsior may prove to be of benefit in select cases such as older donors and non-heart-beating donors in whom the microcirculation may be compromised.44 IGL-1 is a low-viscosity solution that may be superior to UW for the preservation of steatotic grafts.42Although UW still remains the leading preservation solution for livers, “a la carte” use of preservation solutions in specific situations is an attractive option until further studies clarify the benefits of each preservation solution. However, no evidence for the superiority of this approach has been proven. This will be an important field of research with possible implications for procurement-specific practices.
Donors with liver dysfunction
Abnormal liver biochemistry per se do not preclude acceptance of these organs for transplantation.45 Donor liver dysfunction should be evaluated in the context of the donor's general health at the time of organ offering, along with the preceding medical history. Very high levels of transaminases probably indicate a recent ischemic insult; commonly due to hypoperfusion or hypoxia that is seen in patients with cardiorespiratory arrest. The time elapsed between the primary ischemic insult and donor organ offering is of great importance: provided circulation and oxygenation are restored by means of adequate resuscitation, the liver has a greater potential for recovery and liver dysfunction is likely to improve with time. Therefore high transaminase levels in the donor should not be a reason to refuse such liver grafts. More importantly, the absence of metabolic disease or younger age may be considered in favor of using such grafts. The synthetic capacity of the liver is a useful way of analyzing estimated graft function post LT, and prothrombin time/international normalized ratio and bilirubin should be considered surrogate markers along with high transaminases. Metabolic acidosis in the presence of abnormal liver biochemistry is generally an unfavorable combination and liver grafts from such donors are more likely to result in inferior outcomes. Steatosis or fatty liver is also widely prevalent. Hepatic steatosis is frequent in deceased organ retrievals and live donors, and reported in 9%–26% of donors.46, 47, 48 Given the steady increase in the mean age of diseased donors and the overall increase in the prevalence of obesity, it is expected a further increase in the prevalence of steatosis in both deceased donors and living donors.49 The literature suggests poor outcomes following LT using grafts with moderate or severe steatosis.50 Liver dysfunction resulting from any of the immediate pre-donation events mentioned above, on the background of a steatotic liver has a synergistic effect. With the added graft damage from ischemia reperfusion injury such grafts are more likely to fail. Careful evaluation of liver function is therefore important, and in the absence of a severe pre-morbid history even grafts with some degree of liver dysfunction can be used with caution.51 There are no definite guidelines on the upper limit of acceptable abnormal biochemistry. A downward trend in liver enzymes is very important in making such a decision therefore repeated blood tests at least 12 h apart from each other will be an advantage. It is likely that with advanced liver graft preservation techniques currently introduced into transplant practice even grafts with severe dysfunction prior to donation may be resuscitated.
Elderly donors
Studies have proved that organs from younger and “healthy” donors have better outcomes. Donor age is traditionally considered a key parameter predicting graft function. With improved health care the average life expectancy in society is increasing, hence the average donor age is significantly higher than in the past. Donor age per se should not be used as a surrogate for organ quality, at least in liver transplantation. Elderly donors alone should not be considered a contraindication and donor age should be evaluated against the general health of the donor prior to organ donation. Absence of metabolic disease, especially diabetes or hyperlipidemic status is probable indicators that the donor lived a healthy life prior to the clinical event culminating in organ donation. Recent literature also supports the use of liver grafts from upper extremes of age.52 With careful evaluation of the donor history it should be possible to “accept” the best quality donors from this donor population of advancing age.53, 54
Donors with malignancy
The incidence of cancer in donors is approximately 3%, and the risk of transmitting malignancy by transplantation of an organ is roughly 0.01%.55, 56, 57 It can be reasonably assumed that the risk of malignancy increases with donor age, and this means that transplanting organs from elderly donors may increase the risk of transmitting defined and undefined malignancies. Independent of the organ transplanted the most frequently transmitted malignancies originate from central nervous system tumors, melanoma, renal cell carcinoma, and lung carcinoma. The risk of transmission is increased in the case of a metastatic malignancy in donors. In addition, tumor grade is an important risk factor, poor differentiation being associated with a higher risk of transmission.57 Donors with documented history of malignancy are not necessarily discarded. Donors with low-grade malignancies treated years ago (other than melanoma, hematological malignancies) or donors with low-grade central nervous system tumors and an especially low risk of transmission to the recipients may be considered. Guidelines and practices vary according to different countries.58, 59 However, any metastatic malignancy in the donor should exclude donation. Recipients of donors with malignancies should have their immunosuppression modulated because over immunosuppression reduces immune surveillance that can accelerate tumor growth. The potential benefit from mammalian target of rapamycin inhibitors (mTOR), which have both immunosuppressive and antiangiogenic properties,60 requires investigation.
Donors with infection
Infections carry an increased risk of transmission through organ donation and transplantation. If the causative organisms and antibiotic sensitivity patterns of most bacterial infections are known prior to organ donation, and if donors are partly treated, the risk of bacterial transmission and sepsis in the recipient is minimal.61 Meanwhile it is also difficult to estimate the real risk of bacterial sepsis in the recipient that is attributed to the organisms transmitted from the donor. However, there are exceptions to accepting organs from infected donors, as in case of active tuberculosis at the time of donation and a history of Creutzfeldt-Jakob disease. Severe systemic bacterial sepsis concomitant with abnormal liver function are an unfavorable combination, whereas other bacterial diseases, though rare, carry a risk of transmission and the resulting infection may also have implications on graft function (e.g., toxoplasmosis, syphilis).62 These grafts may be used with caution with appropriate post exposure antibiotic prophylaxis or treatment in the recipient.
Historically, human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV) were considered absolute contraindications for organ donation.63 Approximately 5% of people worldwide are chronically infected with hepatitis B. Overall, 15% of those chronically infected go on to develop cirrhosis, and an additional 20% will require LT. Acquisition of the HBV remains a concern after LT because the majority of the infections occur via transmission by the donor liver,64 but some donors with past exposure to HBV infection can be used selectively in some recipients. The development of combined prophylaxis with hepatitis B immune globulin (HBIg) and lamivudine has proved effective not only against HBV recurrence but also against de novo HBV infection or transmission in recipients of anti-HBcAb + livers.65, 66, 67, 68 Nery, et al,68 reported that of 62 recipients of anti-HBc + livers, 60 were serologically free of HBV infection under combined or lamivudine monotherapy. These data suggest that the use of HBcAb + grafts is comparable with core antibody negative grafts and that survival was improved with dual immunoprophylaxis.65, 66, 67, 68, 69 In addition, Prieto, et al,70 reported that post-transplantation HBV infection developed in 15 of 30 recipients of livers from anti-HBcAb + donors compared with 3 of 181 livers from anti-HBcAb — donors. Recipients of livers from anti-HBc + donors are at high risk for acquiring HBV infection, whereas recipients of livers from anti-HBs + donors are significantly less likely to acquire HBV infection, and this latter group may play a role in expanding the donor pool.70
About 5% of all potential organ donors are positive for antibody to HCV,71 and the transplantation because of HCV cirrhosis has increased because of the greater prevalence of the virus in the last 15 years.72 Initially, the use of HCV + donor organs in LT was a source of great controversy and not commonly practiced. Underlying this practice was a concern for increased risk of aggressive viral recurrence in patients receiving HCV + grafts. LT for recipients with HCV cirrhosis from HCV + donors were found to provide graft survival that is equivalent to HCV–grafts to HCV + recipients.73 Short-term studies in the early 1990s showed no difference in outcomes of HCV + grafts; increasing donor shortage allowed for the use of HCV + donor grafts in recipients with HCV to expand the donor pool. Long-term follow-up in the late 1990s confirmed that the use of grafts from HCV + donors is safe and that patient and graft survival are not affected.74 Recurrence rates of hepatitis C, manifested by mild chronic hepatitis, fibrosis, or cirrhosis have been reported to be 54.55% in HCV + donor grafts when compared with 41.74% in HCV–grafts. Patient and graft survival at 4 years post-transplantation in HCV + donor grafts have been shown to be 83.9% and 71.9% versus 79.1% and 76.2%, respectively, in HCV–donor grafts.75 Similar rates of HCV recurrence, patient survival, and graft survival have been reported by different centers using HCV + liver grafts for patients requiring transplantation for HCV cirrhosis.74 Moreover, a report by Marroquin, et al,74 showed that patient survival at two years was significantly higher in HCV + recipients of HCV + grafts than in HCV + recipients of HCV − grafts (90% versus 77%). In contrast, other studies indicated that in patients with HCV-related liver disease, there was no significant patient survival difference between the patients who received HCV + grafts and who received HCV − grafts.75 However, caution should be used in selecting donors with active HCV infection or anti-HCV antibodies. Undetected fibrosis may lead to early graft failure and is also a predictor of HCV recurrence. Liver graft biopsy, histology activity index inflammatory grade and fibrosis scoring are helpful in decision-making.
There have been recent concerns over human T-lymphotropic virus (HTLV)-1 and HTLV-2 transmitted through transplanted and there is reluctance among the transplant community to accept grafts from such donors. It is worth emphasizing that in almost all such cases the donors are “screened” with a serological test.76
Donation after cardiac death
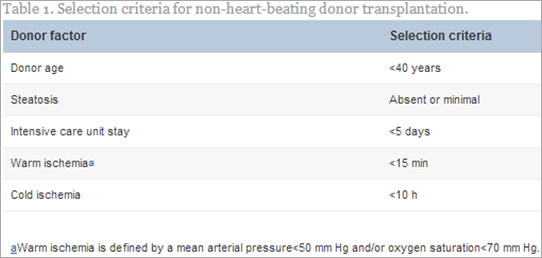
LT from non-heart-beating donors, now termed donation after cardiac death (DCD), is a promising way to increase the supply of organs.77 In controlled circumstances, the organs are retrieved after a standoff period of 2–5 min after death is certified. In either controlled or uncontrolled DCD situations, the organs are subjected to a variable period of warm ischemia, which predisposes them to primary nonfunction, delayed graft function, or irreversible ischemic like diffuse cholangiopathy.78 In early reports, the prolonged period of warm ischemia resulted in markedly increased early graft dysfunction in comparison with donation after brain death donors. Ischemic time has been shown to be extremely important when DCD is considered.79 If warm ischemic time is restricted to <30 min and cold ischemia time <10 h, graft survival rate in the DCD group was found to be 81% and 67% at 1 and 3 years, respectively, which is not significantly different from recipients of dead-brain donors.80 Results from uncontrolled DCD were less good, being graft survival at 2 years of 55%. The use of uncontrolled DCD livers was also associated with significantly higher incidence of PNF, DGF and biliary complications.18, 80, 81, 82, 83, 84 It has been possible to achieve good results with an incidence of PNF below 15% and a lower incidence of biliary complications with specific measures (Table 1).85, 86 These measures include judicious donor selection, including donor age below 40 years and no steatosis, a specific resuscitation technique, including preservation of the organ with systemic heparin, the use of extra corporeal oxygenation, a short warm ischemia time (less than 15 min), and a short CIT (less than 10 h).87, 88 Although this procedure is limited to selected centers with specific protocols, DCD has the potential to increase the donor pool by 10%–20%.89, 90 Methods to address the microcirculation of the biliary system in DCD donors may improve the incidence of biliary strictures.44
Hypernatremia
Hypernatremia has been shown to be one of the variables with prognostic value in predicting graft survival after transplantation in deceased donor liver transplant.91 Some studies have suggested that donors with hypernatremia can affect graft function and increase the risk of graft loss.92 The mechanism for the deleterious effect of elevated donor sodium on graft function is thought to be a result of cell swelling, increased osmolality and exacerbation of reperfusion-mediated injury.83 The cause of hypernatremia could be related to derangement of fluid balance and diabetes insipidus in potential donors.92 In a study investigating the peak donor sodium level and the corrected sodium level at the time of retrieval, it was found that hypernatremia (sodium >155 mEq/L) was associated with 18.5% rate of PNF compared with 3.4% in eunatremic group. With the correction of hypernatremia before procurement, this rise in the PNF was no longer found.83 Another pilot study at University of California examined the effects of infusing 5% dextrose in water through the inferior mesenteric vein before harvesting the organ if the donor sodium level was >160 mEq/L. In the 17 donors who received 5% dextrose to decrease hypernatremia, the rates of DGF/PNF were 0% compared with a group of historical controls that experienced a 60% incident of PNF/DGF.83
Donors with hypotension and inotropic support
Previous UNOS data have shown that donor organs subjected to prolonged hypotension have no significant increase in post-transplantation graft loss. However, graft loss was increased in liver transplant recipients when donors received norepinephrine.92 In other studies, dopamine dose >10 μg/kg/min93 or 6 μg/kg/min83 had a significant effect on early graft function. However, other factors such as age and fat content may modify these effects in either direction.
Briceño, et al,92 reported that unstable donors with high-doses of inotropic drugs have an increase in severe preservation damage rate, and trends to normalize hemodynamic status in dead-brain donors did not correct liver dysfunction. Probably, time-dependent administrations of high-dose dopamine and epinephrine have a harmful effect on liver function.
Split liver grafts
In an attempt to expand the size of the donor pool, a number of surgical techniques have been developed over the past 15 years, including split liver transplant (SLT) and living donor liver transplant (LDLT).94 Couinaud's95 anatomical classification which was later refined by Bismuth,96 permits the creation of partial liver grafts from either deceased or living donors. Surveys in Western populations indicate that SLT in adults is associated with significant increase (about 10%) in graft failure and recipient morbidity.8, 33, 97, 98, 99 Results are notably better in children.100 Even if split liver grafts are procured from young donors with normal parenchyma and short CIT, they should be considered extended criteria grafts for the following reasons: (a) the graft volume is generally lower than the recipient's standard liver volume and may be insufficient to adequately meet the metabolic demand during the early postoperative course. (b) There are higher technical requirements, and nonoptimal positioning of the partial graft may result in compromised venous outflow. As a result, biliary leakage, hepatic artery thrombosis, focal or outflow obstruction, and poor early graft recovery are more frequent in comparison with whole organ transplantation.101 SLT for 2 adults has been performed in select transplant centers with better results for right allografts versus left allografts.102, 103 Adult transplantation with a left graft remains a challenging technical procedure with a high risk of primary nonfunction due to insufficient parenchymal volume and often complex biliary and vascular anastomosis.101 Unless significant technical advances are achieved, the use of left allografts cannot be widely applied to adults but are best suited for pediatric recipients in whom SLT offers excellent results. In adults, SLT using the right lobe marginally increases the rate of graft failure. This should not represent a disincentive for using SLT, as this technique expands the donor pool, particularly for pediatric recipients.
Our experience
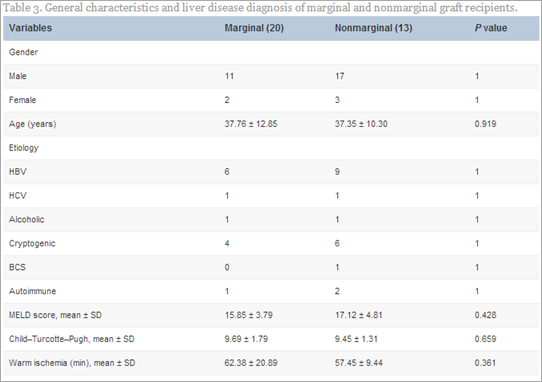
We analyzed our initial results of extended criteria donors for DDLT.104 A total of 33 patients were enrolled in this study. There were 20 marginal and 13 nonmarginal grafts. The two groups were well matched regarding age, sex and indication of liver transplantation, model for end-stage liver disease score, technique of transplant, requirement of vascular reconstruction, warm ischemia time, blood loss, mean operative time, etc (Table 2).
In our study, posttransplant peak level of liver enzymes, international normalization ratio, and bilirubin were not statistically significant in the marginal and nonmarginal group. Wound infection occurred in 10% of marginal compared with 7.7% of nonmarginal graft recipients (P > 0.05). In the marginal group, the incidences of vascular complications, hepatic artery thrombosis (four), and portal vein thrombosis (one) were not statistically significant compared to the nonmarginal group. Acute rejection was observed in a total of seven patients (21.2%)—five (25%) in the marginal group and two (15.4%) in the nonmarginal graft recipients. Primary nonfunction occurred in three (9.1%) patients—two (10%) in the marginal and one (7.7%) in the nonmarginal group. Average patient survival for the whole group was 91% at 1 week, 87.8% at 3 months, and 84.8% at 6 months (Table 3).
Ethical considerations and informed consent
An ethical allocation practice is required based on justice, equity, and utility. Prospective recipients must be informed about the possibility of allograft-specific risks. They need to understand early in the transplant process (ideally at listing and without an allograft available) that donor risk is a continuum. Risk of graft failure and risk of disease transmission should also be emphasized as part of the informed consent process. Criteria for accepting and discarding extended criteria donors should be laid down in each transplant centre. A prospective evaluation necessitates that the donor characteristics and the outcome should be periodically reported in a standardized manner and centralized.
Future perspectives
Use of normothermic regional perfusion (NRP) is under evolution. Principally, it is the amalgamation of two strategies to improve donor organ quality: Extra corporeal membrane oxygenation (ECMO) and in situ organ perfusion.105 In the case of DCD organs it may be correct that if the inferior outcomes of DCD grafts are indeed due to initial donor warm ischemia times, these adverse insults can be counter acted by attempts to resuscitate organs (upon cardiac death) using ECMO.106However, provided the cerebral circulation is isolated in the donor, the donor blood vessels provide the conduits for inflow and outflow to the ECMO circuit. In this sense it differs from ex situ perfusion, and organs are revived “in situ”. The evidence for success comes from Spain, where similar interventions have been employed in different organ donor settings where NRP has been successfully performed in the uncontrolled DCD setting.107, 108 As mentioned above, the practice of NRP is still in its infancy and none of the pioneering centers have yet published their results but the future of marginal DCD organ utility may rely on this novel intervention.
Conclusion
The issue of imbalance between the number of potential recipients of liver transplantation and available donors will not be resolved within the next few decades. So a number of patients with end-stage liver disease and/or liver malignancy will not considered for liver transplantation, although they could derive a significant benefit from this option. Therefore, in the absence of an efficient alternative to transplantation, the expansion of the donor pool will continue to be a priority. Moreover, ideal donors are often rare and most of the time the prospective donor has one or more adverse factors that make it an ECD. An ECD graft therefore should be regarded as an “otherwise unused” graft; hence it should benefit any recipient provided it is in favor of the recipient both in terms of survival and disease transmission. So-called ECD therefore have to be entertained to increase the graft utility rate. By employing a “utilitarian” approach, a significant number of organs from unconventional donors may be used. They may offer two benefits. Firstly, a utilitarian approach should be adopted to choose the best recipient to benefit from the particular graft in question, and secondly this may relax the competition in the transplant wait list.
Conflicts of interest
All authors have none to declare.

No comments:
Post a Comment