Clin Infect Dis. (2014)doi: 10.1093/cid/ciu012
First published online: January 6, 2014
This article is Open Access
Andrew Hill1, Saye Khoo1, Joe Fortunak2, Bryony Simmons3, Nathan Ford4
1 Department of Pharmacology and Therapeutics, Liverpool University, UK
2 Chemistry and Pharmaceutical Sciences, Howard University, Washington, DC, USA
3 Imperial College London, London, UK
4 Centre for Disease Epidemiology and Research, University of Cape Town, South Africa
Address for correspondence: Dr Andrew M Hill PhD, Senior Visiting Research Fellow, Department
of Pharmacology and Therapeutics, University of Liverpool, 70 Pembroke Place, Liverpool L69 3GF,
United Kingdom. Email: microhaart@aol.com
Summary: Large-scale manufacture of treatment to cure Hepatitis C is feasible, with target prices of US$100-250 per 12 week treatment course. These low prices could make widespread access to
HCV treatment in low and middle income countries a realistic goal.
Abstract
Background: Several combinations of two or three Direct Acting Antivirals (DAAs) can cure HCV in
the majority of treatment-naïve patients. DAAs for HCV infection have similar mechanisms of action
and chemical structures to antiretrovirals for HIV infection. Generic antiretrovirals are currently
manufactured at very low prices, to treat 10 million people with HIV/AIDS in developing countries.
Methods: Four HCV DAAs, currently either in Phase III development or recent approval (daclatasvir,
sofosbuvir, simeprevir, faldaprevir) and ribavirin were classified by chemical structure, molecular
weight, total daily dose and complexity of synthesis. The likely range of manufacturing costs per
gram of DAA were then projected as formulated product cost, based upon treating a minimum of
one million patients annually (to arrive at volume demand) combined with an analysis of the
complexity of synthesis and a 40% margin for formulation. Projections were then compared with
actual costs of antiretrovirals with similar structures.
Results: Minimum manufacturing costs of antiretrovirals were US$0.2-2.1/g. The complexity of
chemical synthesis for HCV DAAs was ranked from lowest to highest: ribavirin, daclatasvir,
sofosbuvir, faldaprevir and simeprevir. Predicted manufacturing costs for 12-week courses of HCV
DAAs were: US$21-63 for ribavirin, US$10-30 for daclatasvir, US$68-136 for sofosbuvir, US$100-
210 for faldaprevir, and US$130-270 for simeprevir.
Conclusions: Within the next 15 years, large-scale manufacture of two or three drug combinations of HCV DAAs is feasible, with minimum target prices of US$100-250 per 12 week treatment course. These low prices could make widespread access to HCV treatment in low and middle income countries a realistic conclusion.
Introduction
Worldwide, over 185 million people are infected with Hepatitis C Virus (HCV), with up to 500,000 HCV-related deaths per year.1,2 The vast majority of these patients are left untreated, with treatment rates ranging from 3.5% in Europe to 21% in the US.3,4
In comparison, approximately 35.3 million are infected with Human Immunodeficiency Virus (HIV), with 1.6 million HIV-related deaths per year.5 The vast majority of these infections and deaths are in resource-limited settings. Due to remarkable progress in reducing costs of treatment, over ten million patients are now on antiretroviral (ARV) regimens in low- and middle-income countries.5
People with Hepatitis C infection can be cured using modern treatment, but at a very high cost. The US launch prices for 12 weeks treatment with simeprevir and sofosbuvir, two newly approved drugs, are US$66,000 and US$84,000, respectively.6 In low and middle income countries, access to HCV treatment is extremely limited, mainly because of the complexity of patient management and high costs.7 Of the twenty countries with the largest HCV epidemics, twelve are classified as low or lower-middle income (Table 1). For widespread treatment of HCV in developing countries to become feasible, we will need short-course antiviral treatment available at very low costs and with minimal diagnostic support.
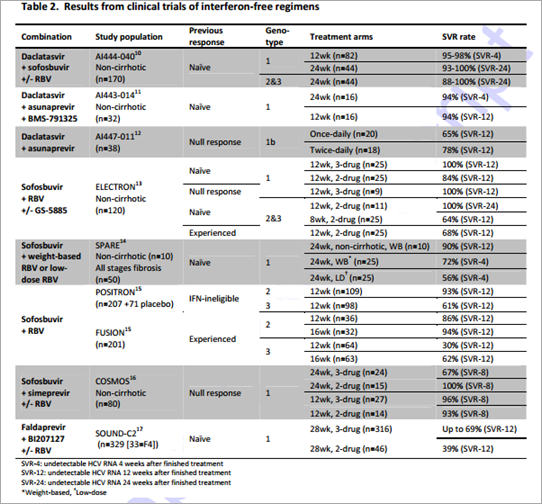
Recently, a number of direct acting antivirals (DAAs) in Phase 2 or 3 development have shown sustained virological response (SVR) rates of up to 100% in non-cirrhotic patients and, promisingly, high SVR rates in people with advanced liver disease or previous null response (Table 2). The approval of such drugs will likely see DAA combinations replace interferon-based regimens as the new standard of care.18 Success of treatment often depends on genotype and while the majority of results are from clinical trials carried out on patients infected with the HCV-1 genotype, encouraging results are emerging for the treatment of both HCV-2 and HCV-3 (Table 2).
DAAs for HCV infection have similar mechanisms of action and chemical structures to antiretrovirals currently in use for the treatment of HIV. These drugs are also similarly intended for oral delivery using relatively uncomplicated formulation technologies. Over the last two decades, generic competition, increased purchase volumes, and improvements in manufacturing processes (both API and formulation) have driven the cost of HIV antiretroviral treatment down by over 99%, with standard triple therapy now costing as little as US$60 per patient per year.19
These prices could not have been imagined when triple antiretroviral drug combinations were introduced at over US$10,000 per patient/year in the late 1990s.7 Following the important precedent set by access to treatment for HIV infection, the aim of this analysis was to estimate the minimum cost of HCV treatment, assuming the same strategic market dynamics as used to supply antiretrovirals to people with HIV/AIDS in developing countries.
Methods
In this analysis, the molecular structures of HCV DAAs were compared with their closest analogues in the treatment of HIV. We evaluated the likely routes of manufacturing as published by the originator companies and assumed a volume demand based on 1-5 million patients per year to arrive at approximate costs of DAA active pharmaceutical ingredients (APIs). We then added on a 40% margin for finished production manufacturing (formulation) to arrive at a projected cost of therapy.
The purpose of this analysis is to logically speculate whether DAAs can be provided for millions of people at a reasonable cost. The analysis is not meant to be exact or to arrive at a “most likely optimised cost” for any individual or combination DAA therapy. Very little information is presently available to estimate actual commercial formulation costs for DAAs. These DAAs are all delivered orally using conventional technologies. Projected API costs for these DAAs range from US$1,400- 21,000/kg; as such the very high relative costs of API would justify a 40% increment as a reasonable add-on for estimating cost of the finished dosage form.
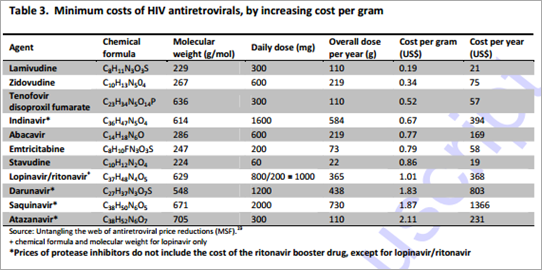
The minimum treatment costs of comparator HIV antiretrovirals were calculated using the lowest prices reported by manufacturers to Médecins Sans Frontières in 2012.19 Minimum costs per gram of ARVs ranged from US$0.20 to US$0.90/g for nucleoside analogues, US$0.50/g for nucleotide analogues, and US$0.70-2.10/g for protease inhibitors (Table 3).
Four HCV DAAs were included in this study: daclatasvir (Phase III), sofosbuvir (approved December 2013), faldaprevir (Phase III), and simeprevir (approved November 2013). Ribavirin, already available as a generic, was also included in this analysis due to its likely inclusion in future drug regimens. Using the most likely daily dosage identified from clinical trials, the total drug requirement for each DAA was calculated for a 12 week course of each HCV DAA. In order to estimate the manufacturing cost of each HCV DAA, current routes of synthesis and critical cost-limiting raw materials were taken from the patent literature. In an alternative comparison, each compound was matched to the closest equivalent HIV ARV based on structural similarity.
Production costs per gram of HCV DAA were assumed to be 1-10 times higher than the equivalent HIV ARV dependant on the complexity of chemical synthesis. Complexity was assessed by identifying the most likely cost-limiting intermediates for the synthesis of each DAA. Additional considerations included ease and number of steps of manufacture and availability and cost of starting materials. Using this estimate for the production cost per gram of drug together with the total drug requirement, an estimate for the minimum cost of a 12 week course of treatment with each HCV DAA was calculated. These costs were used to provide estimates for the production costs of 2- or 3-drug combination therapy based on the combinations currently being investigated in clinical trials (Table 2)
Results
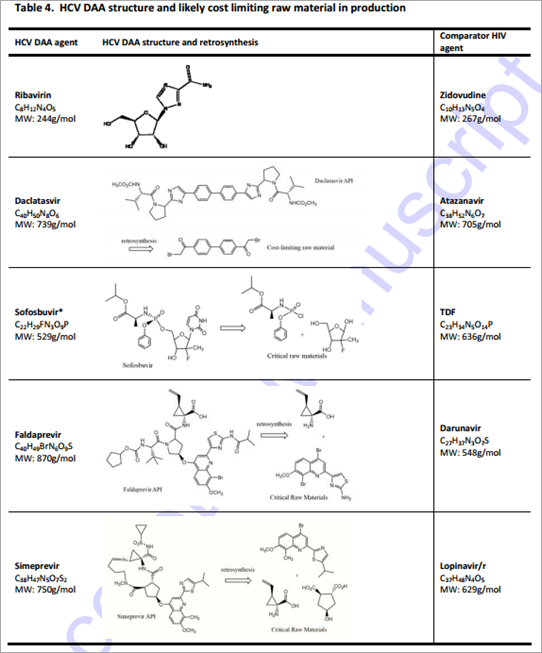
Based on molecular weight, chemical structure, class, and dose, the HIV ARV most comparable to each HCV DAA were as follows: zidovudine to ribavirin, atazanavir to daclatasvir, tenofovir and stavudine to sofosbuvir, darunavir to faldaprevir, and lopinavir to simeprevir. Table 4 shows the HCV DAA and most comparable HIV antiretroviral as well as the likely cost limiting raw materials in production. A summary of the estimated costs per person for a 12 week course of each HCV DAA is shown in Table 5
Ribavirin With a daily dose of 1000-1200mg dependent on patient weight, a 12-week course of treatment with ribavirin will require between 84.0 and 100.8 grams of API. This results in a range of demand between 84 and 504 metric tonnes of API to treat between 1-5 million patients. Ribavirin is a nucleoside analogue with a molecular weight of 244g/mol. Based on this and the chemical formula (Table 3), zidovudine was considered to be the closest equivalent HIV relative of ribavirin (also a nucleoside analogue with molecular weight 267g/mol). Analysis revealed that ribavirin has a relatively simple chemical synthesis.20 Using zidovudine alongside knowledge of the current costs of ribavirin, production costs were estimated to lie between US$0.26 and US$0.41 per gram, giving potential costs for a 12-week course of ribavirin of US$34-48 for the dose of 1000mg per day, and US$41-58 for the dose of 1200mg/day.
Daclatasvir At the dose of 60mg/day, a 12-week course of treatment would require 5.0 grams of daclatasvir API. Daclatasvir is a NS5A inhibitor with molecular weight 739g/mol. Treating 1-5 million patients with daclatasvir would require 5-25 metric tonnes of API. Daclatasvir was deemed most structurally comparable to atazanavir, a protease inhibitor with molecular weight 705g/mol (Table 3). Daclatasvir has a straightforward synthesis with the cost-limiting intermediate being the substituted biphenyl compound shown (Table 5).21,22 There is wide availability of cheap starting materials to synthesise the side-chains of daclatasvir. The estimated production costs of daclatasvir finished product are between US$2 and US$6 per gram. At a daily dose of 60mg, the estimated production costs for a 12-week course of treatment were between US$10-US$30.
Sofosbuvir Delivered at a 400 mg daily dose, a 12-week course of treatment with sofosbuvir will require 33.6 grams of API. This results in a range of API demand between 33.6 and 168 metric tonnes to treat 1-5 million patients. With a molecular weight of 529g/mol and chemical formula of C22H29FN3O9P, sofosbuvir was considered most structurally comparable to tenofovir, with a molecular weight of 636g/mol and chemical formula C23H34N5O14P (Table 3). The cost-limiting raw material/intermediate for the synthesis of sofosbuvir API is the 2’-fluoro-2’-methylfuranose intermediate (Table 5). 23,24,25 Although a number of approved drugs have similar structures, the presence of both a methyl and a fluoro substituent at the 2’ position makes this intermediate cost-limiting. We have estimated API production costs for sofosbuvir between US$2 and US$4 per gram; sofosbuvir is considered most closely analogous in cost to the HIV drug stavudine, which is relatively expensive in terms of manufacturing costs per gram. An estimated cost for 12 weeks of treatment with sofosbuvir API is US$68-136.
Faldaprevir At a daily dose of 120 mg, 10.1 grams of faldaprevir API will provide a 12-week course of treatment. Treating 1-5 million patients with Faldepravir would require 10.1-50.5 metric tonnes of API. Faldaprevir is a protease inhibitor with a molecular weight of 870g and chemical formula of C40H49BrN6O9S (Table 3). As a result of this high molecular weight and complicated chemical structure darunavir was deemed the most cost-comparable with faldaprevir. Faldaprevir manufacture requires a tetra-substituted quinoline and a vinyl-cyclopropane amino acid as raw materials for the synthesis of the API (Table 5).25,26 Due to the difficult synthesis, estimated production costs of US$10-21 per gram were applied. Accordingly, a 12-week course of treatment with faldaprevir could cost between US$100 and US$210.
Simeprevir At a daily dose of 150 mg, a course of treatment with simeprevir would require 13 grams of simeprevir API. Treating 1-5 million patients with Simepravir would require 13-65 tonnes of API. In terms of class, molecular weight and chemical structure, lopinavir/r was considered the equivalent HIV ARV of simeprevir, although atazanavir was included in comparing cost estimates (Table 3). Simeprevir is a medium-ring macrocycle that utilises a ring-closing metathesis reaction in the late stages of API manufacturing, which is challenging (Table 5). Novel raw materials entered into the synthesis include a tetra-substituted quinoline and the same vinyl-cyclopropane amino acid as used in the synthesis of faldaprevir.27 Production costs were estimated at US$10 to US$21 per gram, giving an estimated cost of treatment of US$130-270 for 12 weeks.
Combination therapies Table 6 shows the estimated prices of combination therapy based on the DAA drug prices calculated in this analysis. Using the minimum costs, a 12-week course of treatment with daclatasvir and sofosbuvir could cost a minimum of US$78 per person. A treatment with sofosbuvir and simeprevir could cost US$198 for 12 weeks with the addition of ribavirin increasing this cost to US$219 per person. For some patients or some regimens, a 24-week treatment might be necessary, doubling treatment costs.
Discussion
Fifteen years ago, universal access to antiretroviral therapy for HIV/AIDS in developing countries was considered too complex and expensive to be feasible. With the invention of effective, simple therapy, a market was created for generic competition that in turn made treatment affordable, and along with the associated international funding, treatment scale up became possible.28 The situation of HCV treatment today is reminiscent of treatment for HIV/AIDS in the year 2000.
Our analysis suggests that 2 or 3 drug combinations of interferon-free HCV treatments could cost US$100-250 for a 12 week course of treatment. These low prices coupled with the high SVR rates established in several trials shows the potential for large-scale, low cost HCV treatment in developing countries, with the potential to repeat the model of low-cost HIV treatment that has benefitted millions of people. This model of treatment is based on simplified, standardised treatment approaches using tolerable, easy-to-administer regimens that are supportive of task shifting (care delivery by lesser trained health staff) and good patient adherence, and could be facilitated by widespread access to oral, short-course DAA therapy in low income countries.29
The cost of production of HIV antiretrovirals has fallen progressively over the past decade through increased market competition, increased volumes, and efficiencies in manufacturing processes. The Clinton Health Access Initiative pioneered purchasing from quality-assured generic pharmaceutical manufacturers in India and raw material manufacturers in China to ensure the lowest sustainable costs for some antiretrovirals, lowering prices dramatically.30 Our estimated unit costs of HCV DAAs per gram are still far higher than the current costs of HIV antiretrovirals, and it could be assumed that the cost of HCV DAAs may further decrease over time through process optimisation and the cheaper sourcing of raw materials as volume demand drives competition and process efficiencies.
We have been conservative in our estimations versus the history of costs for HIV drug production. API syntheses begin with raw materials of rather simple structure that are combined in a specific and modular fashion to build the more complex structures of drugs. When a commercial market already exists for these raw materials, their contribution to cost is rather modest. When raw materials with no previous commercial demand are used, these can contribute very substantially to cost. Efavirenz, an HIV-1 reverse transcriptase inhibitor, provides an illustration of this. Cyclopropylacetylene (CPA) is a raw material for the synthesis of efavirenz. During human clinical trials, when the demand for CPA was only a few metric tonnes, CPA cost was US$800-1350/kg. When the drug was approved (1998) and demand for CPA was about 50 tonnes per year, the price of CPA had fallen to US$300-350/kg. Today, with a global demand for efavirenz of over 800 tonnes/year, CPA can be purchased for US$50-60/kg. Efavirenz was launched in 1998 at an API cost of $1800/kg. The current best cost for the API is about $120/kg from Indian generic suppliers.
The DAA molecules in this analysis each contain at least one novel raw material that will be expensive in the early phases of commercial introduction. Similar to the reductions in the cost of efavirenz, the estimated costs in this analysis can only be justified if we can guarantee the eventual procurement of large orders. With the DAAs investigated in this study, twelve weeks treatment for one million patients would require between 5 and 34 metric tonnes of API, with ribavirin requiring between 84 and 101 tonnes of active ingredient. Treatment for only a fraction of the 185 million people infected with HCV could ensure order sizes in a similar region to HIV antiretrovirals.
With the introduction of these new HCV DAAs, the methods used to diagnose and monitor HCV are likely to be greatly simplified.31 In order to ensure widespread treatment, the costs of diagnostic and monitoring tests will also need to fall. In the same way that the cost of HIV treatment has decreased, the cost of HIV diagnostics and monitoring has rapidly declined over the last few decades with commercial HIV RNA tests now costing less than US$2 per test.32
There are several limitations with this analysis. Firstly, in order to calculate more precise costs, more detailed analysis of the API and formulation processes for production will be necessary. Very little specific information is currently available about formulations of the DAAs. However, all of the DAAs are for oral delivery and – to the best available knowledge – use relatively common drug release technologies. This is in parallel with the formulation of ARVs. Given this, we applied a 40% conversion cost of API to finished product, noting that this figure is not exceeded for any of the large-scale ARV combinations being marketed. Secondly, access to the HCV DAAs at minimum prices in developing countries will strongly depend on the level of enforcement of patent restrictions. These price estimates are based on the previous experience with production of ARVs for HIV treatment which assumes market competition through generic manufacture. Legal mechanisms such as voluntary or compulsory licenses may be needed to overcome patent barriers and stimulate such competition. Patents for daclatasvir,33 sofosbuvir,34 faldaprevir,35,36 and simeprevir will remain in force until at least 2025,37 After which time it should be possible to produce generic versions at much lower cost, provided no additional patents are granted for modifications such as route of synthesis, crystalline structures or methods of use. Such ‘evergreen’ patenting has happened repeatedly in HIV drug development and could further delay the introduction of generic DAAs.19,38 In the near future, there need to be negotiations with the patent holders on voluntary licensing, access prices for low and middle income countries, and mass production of low cost DAAs. Unless these DAAs are widely introduced, current death rates from HCV of 500,000 people per year will continue for many years to come.
Finally, our analysis is limited to HCV DAAs that have been mainly evaluated in genotype 1 HCV. Although sofosbuvir has recently been approved in combination with ribavirin for the treatment of genotypes 2 and 3, further research needs to be conducted to ensure pan-genotypic coverage of HCV.
The high cost of drugs is often justified by the need to recover costs of research and development (R&D). In the case of antiretrovirals for HIV, many of these costs were assumed by the public sector, where parts of the drug discovery and development occurred.39 Several mechanisms have been used by originator companies to allow access to antiretrovirals in low-income settings. These include differential pricing (charging more in high-income countries) and voluntary licensing (allowing third party generic manufacture). Similar mechanisms could be employed for HCV DAAs to ensure these drugs are affordable, while also providing a financial return on the costs of research and development.40 Commitments by national governments to scale up antiretroviral therapy, with support from international donors, were also critical to leveraging prices by increasing the size and predictability of the HIV market. This will be an essential factor in lowering drug prices and increasing access to HCV DAAs.41
Expanding access through greater affordability will also confer indirect benefits. Currently a large proportion of untreated patients continue to spread the HCV pandemic worldwide. Lessons learnt from HIV suggests that with the introduction of strong community programmes for testing, and high rates of treatment coverage and retention, expanded access to treatment is also likely to have a pronounced effect on HCV transmission, a benefit already suggested in modelling studies.42
Widespread access to HCV DAAs will also require fast regulatory approvals of new drugs, political will, establishment of national viral hepatitis programs, early access programs to start treating people who are most at risk, and accepted international HCV treatment guidelines.
In conclusion, widespread access to combinations of HCV DAAs is feasible, with potential target prices of US$100-250 per person for a 12 week treatment course. Progressive reductions in these costs are likely through optimisation of chemical synthesis and cheaper sourcing of raw materials. These low prices could make widespread access to HCV treatment in low and middle income countries a realistic goal, with substantial individual and public health benefits.
Source of funding: There was no funding provided for this project. All the authors contributed their time and resources voluntarily.
Conflicts of interest: JF, BS and NF report no conflicts of interest. AH has received consultancy payments from Janssen, not connected with this project. SK has received consultancy payments and travel grants from pharmaceutical companies, not connected with this project.
Authors’ contributions: Andrew Hill wrote the original plan for analysis and coordinated the project. Bryony Simmons conducted the literature search and compiled the data for analysis. Joe Fortunak worked on the routes of chemical synthesis and raw materials. Nathan Ford worked on the patent access issues. Saye Khoo worked on the clinical issues in treatment of Hepatitis C. All authors critically reviewed the manuscript and approved the final version.
Additional figures see pages 24-28 of PDF
References (See pages 20 – 23 of PDF)
* Sofosbuvir is presumably synthesized as a mixture of diastereomers on phosphorous. Only one f these two diastereomers is desired in the API. Separation of diastereomers can be a substantial contributor to the cost of API production, particularly when an undesired diastereomer cannot be fficiently recycled. See US Patent 7,390,791 and US Application 20130065856 for the tenofovir alafenamide fumarate (TAF) prodrug version of tenofovir which contains an identical “ProTide™” oiety as that present in Sofosbuvir. Efficient recycling of diastereomers has been demonstrated for AF, but we do not presume this is the case for Sofosbuvir.



No comments:
Post a Comment