Clinical Findings Support Initiation of C-EDGE Phase 3 Program
Friday, April 11, 2014 1:00 am EDT
"These findings provide additional clinical evidence regarding the potential of MK-5172/MK-8742 in treating a broad spectrum of HCV patients."
WHITEHOUSE STATION, N.J.--(BUSINESS WIRE)--Merck (NYSE:MRK), known as MSD outside of the United States and Canada, today announced interim results from the ongoing C-WORTHy study, a multi-arm Phase 2 clinical trial evaluating the efficacy and safety of an all-oral, once-daily regimen combining MK-5172, an investigational hepatitis C virus (HCV) NS3/4A protease inhibitor, and MK-8742, an investigational HCV NS5A replication complex inhibitor, among patients with chronic HCV Genotype 1 infection (GT1). Interim analysis of hard-to-cure1 patients administered MK-5172/MK-8742 with and without ribavirin (RBV) for 12 or 18 weeks showed sustained viral response2 (SVR), 4 to 8 weeks after the completion of therapy (SVR4/8):
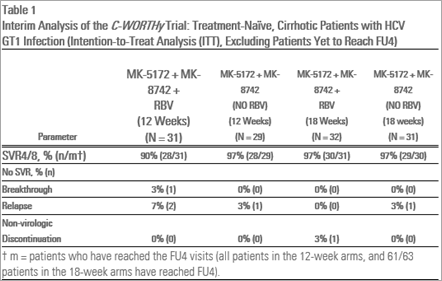
- HCV GT1 infected, treatment-naïve cirrhotic patients, MK-5172/MK-8742 treated - 97 percent (28/29 and 29/30) for 12 and 18 weeks, and MK-5172/MK-8742 plus RBV - 90 percent (28/31) and 97 percent (30/31) for 12 and 18 weeks, respectively.
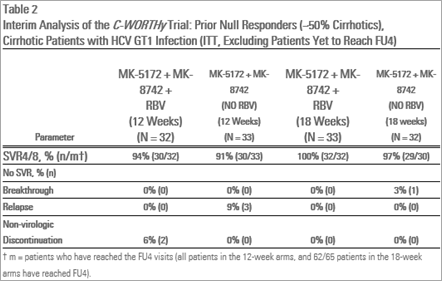
- HCV GT1 infected prior-null responder patients (with or without cirrhosis), MK-5172/MK-8742 treated - 91 percent (30/33) and 97 percent (29/30) for 12 and 18 weeks, respectively, and MK-5172/MK-8742 plus RBV treated 94 percent (30/32) and 100 percent (32/32) for the 12 and 18 weeks, respectively.
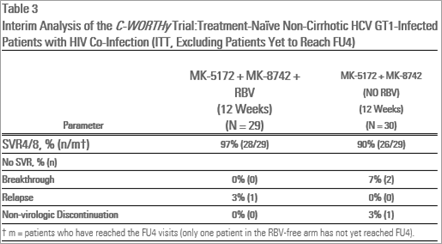
- Treatment-naïve, non-cirrhotic patients with HCV/HIV co-infection, MK-5172/MK-8742 treated for 12 weeks - 90 percent (26/29) and MK-5172/MK-8742 plus RBV for 12 weeks 97 percent (28/29).
These data were presented at the 49th Annual Meeting of the European Association for the Study of the Liver (EASL), also known as The International Liver Congress™ 2014 in London, UK.
“There is still a need for further options for the most difficult-to-cure patients, including those with cirrhosis and HCV/HIV co-infection,” said Dr. Eric Lawitz, MD, vice president, Scientific and Research Development, The Texas Liver Institute, and clinical professor of medicine, University of Texas Health Science Center in San Antonio. “These findings provide additional clinical evidence regarding the potential of MK-5172/MK-8742 in treating a broad spectrum of HCV patients.”
C-WORTHy Study Design
C-WORTHy is a randomized, dose-responsive, parallel-group, multiple-site, double-blind clinical trial comparing different patient populations exposed to different durations of treatment of MK-5172 (100 mg once daily) in combination with MK-8742 (50 mg once daily) with or without RBV in subjects with chronic HCV infection. A total of 471 patients with chronic HCV GT1 and HCV RNA levels of ≥10,000 IU/mL were enrolled in C-WORTHy and randomized across 16 arms. These results examine hard-to-cure subpopulations, including treatment-naïve patients with liver cirrhosis (12- and 18-week arms, with and without RBV), prior-null responder patients with and without cirrhosis (12- and 18-week arms, with and without RBV) and patients with HIV/HCV co-infection (12-week arms).
Key Findings for MK-5172/MK-8742
Viral suppression (HCV RNA levels less than 25 IU/mL) was demonstrated for treatment-naïve patients with cirrhosis, prior-null responder patients with and without cirrhosis and HIV/HCV co-infected patients by Treatment Week (TW)12. These levels were maintained at rates between 90 and 100 percent across patient subgroups through the completion of dosing and at the four-week treatment follow-up time point (FU4).
The most common adverse events observed among treatment-naïve patients with cirrhosis and prior-null responder patients with and without cirrhosis were fatigue (23% and 28%, respectively), headache (24% and 24%, respectively), and asthenia (8% and 19%, respectively). The most common adverse events observed among HIV co-infected patients were headache (8%), asthenia (8%), fatigue (7%), and sleep disorder (7%). There were no early discontinuations due to drug-related adverse events and no clinically significant abnormalities observed in routine laboratory analysis of hematologic markers.
About Merck’s Phase 3 HCV Program: C-EDGE
Based on the results of the Phase 2 clinical program, Merck has initiated Phase 3 clinical trials for MK-5172/MK-8742. The Phase 3 program, called C-EDGE, will evaluate the safety and efficacy of MK-5172/MK-8742 with and without ribavirin in various genotypes and across a broad range of patient populations with chronic HCV. Study cohorts will include: C-EDGE TN (GT1, GT4-6; treatment-naive ± cirrhosis), C-EDGE CO-INFXN (GT1, GT4-6; treatment-naive ± cirrhosis with HIV/HCV co-infection), C-EDGE RECOVERY (GT1, GT4-6; treatment-naive ± cirrhosis; ± HIV/HCV co-infection on opiate substitution therapy), and C-EDGE TE (GT1, GT4-6; prior failed treatment with peginterferon/ribavirin; ± HIV/HCV co-infection). Study information can be found at www.clinicaltrials.gov.
Merck’s Commitment to HCV
For more than 25 years, Merck has been at the forefront of the response to the HCV epidemic. Merck employees are dedicated to applying their scientific expertise, resources and global reach to deliver healthcare solutions that support people living with HCV worldwide.
About Merck
Today's Merck is a global healthcare leader working to help the world be well. Merck is known as MSD outside of the United States and Canada. Through our prescription medicines, vaccines, biologic therapies, and consumer care and animal health products, we work with customers and operate in more than 140 countries to deliver innovative health solutions. We also demonstrate our commitment to increasing access to healthcare through far-reaching policies, programs and partnerships. For more information, visit www.merck.com and connect with us on Twitter, Facebook and YouTube.
Merck Forward-Looking Statement
This news release includes “forward-looking statements” within the meaning of the safe harbor provisions of the United States Private Securities Litigation Reform Act of 1995. These statements are based upon the current beliefs and expectations of Merck’s management and are subject to significant risks and uncertainties. There can be no guarantees with respect to pipeline products that the products will receive the necessary regulatory approvals or that they will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.
Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and health care legislation in the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; Merck’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of Merck’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
Merck undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. Additional factors that could cause results to differ materially from those described in the forward-looking statements can be found in Merck’s 2013 Annual Report on Form 10-K and the company’s other filings with the Securities and Exchange Commission (SEC) available at the SEC’s Internet site (www.sec.gov).
1 Defined as treatment-naïve patients with liver cirrhosis, prior-null responder patients with and without cirrhosis and patients with HIV/HCV co-infection
2 Defined as HCV RNA below the limit of quantification or below the limit of detection at the last visit on record – 4, 8, 12, or 24 weeks after the completion of therapy
Contact:
Merck
Media Contacts:
Caroline Lappetito, 267-305-7639
Sarra Herzog, 201-669-6570 or
Investor Contacts:
Carol Ferguson, 908-500-1101
Justin Holko, 908-423-5088
No comments:
Post a Comment