- OLYSIO™ (Simeprevir) provides a new triple therapy treatment option, as well as the first ever 12-week interferon-free and ribavirin independent treatment regimen, in combination with sofosbuvir, for appropriate patients in Europe
Stockholm, Sweden — Medivir AB (OMX: MVIR) today announces that simeprevir has been granted marketing authorisation by the European Commission (EC) for the treatment of adults with genotype 1 and 4 chronic hepatitis C in combination with other medicinal products.
“The approval of simeprevir in Europe is a further step in our partner's global strategy to enable an improved treatment for hepatitis C patients. This also means that Medivir will now be able to offer this treatment to patients in the Nordic region, where we have the marketing rights“, says Maris Hartmanis, CEO, Medivir.
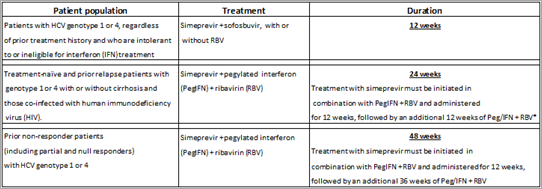
This marketing authorisation represents a significant milestone in the development of new triple therapy hepatitis C (HCV) treatment options for genotype 1 and 4 patients. It also includes simeprevir as part of an all oral 12-week interferon-free direct-acting antiviral (DAA) regimen with or without ribavirin (RBV), in genotype 1 or 4 patients, who are intolerant to or ineligible for IFN treatment.
* Treatment-naïve and prior relapse patients with cirrhosis who are co-infected with HIV should receive 48 weeks of treatment. Treatment with simeprevir must be initiated in combination with PegIFN + RBV and administered for 12 weeks and then followed by an additional 36 weeks of PegIFN + RBV.
The EC approval for simeprevir with PegIFN + RBV is based on a clinical trial programme involving three pivotal phase III studies, with over 1000 patients. The trials; QUEST-1, QUEST-2 and PROMISE, explored the use of simeprevir in combination with PegIFN/RBV in treatment-naïve patients and patients who have relapsed after prior interferon-base treatment. All three studies met their primary endpoints and demonstrated that simeprevir in combination with PegIFN/RBV, achieves significant cure rates when compared with PegIFN/RBV alone.
The EC approval for the combination of simeprevir and sofosbuvir also contains the phase II study, COSMOS. This was based upon prior null responder and treatment-naïve patients.
For more information please contact:
Rein Piir, EVP Corporate Affairs & IR, mobile: +46 708 537 292
Medivir is required under the Securities Markets Act to make the information in this press release public. The information was submitted for publication at 13.00 CET on 16 May 2014.
About Simeprevir
Simeprevir is an NS3/4A protease inhibitor jointly developed by Janssen R&D Ireland and Medivir AB and indicated for the treatment chronic hepatitis C infection in combination with pegylated interferon and ribavirin in HCV genotype 1 and 4 infected patients with compensated liver disease, including cirrhosis.
Janssen is responsible for the global clinical development of simeprevir and has exclusive, worldwide marketing rights, except in the Nordic countries. Medivir AB retains marketing rights for simeprevir in these countries under the marketing authorization held by Janssen-Cilag International NV. Simeprevir was approved for the treatment of chronic hepatitis C infection as part of an antiviral treatment regimen in combination with pegylated interferon and ribavirin in genotype 1 infected adults with compensated liver disease, including cirrhosis in September 2013 in Japan, in November 2013 in Canada and the U.S. and in March 2014 in Russia. Following the EMA approval, it is anticipated that simeprevir will be available across a number of European Union countries in conjunction with reimbursement, in the second half of 2014.
About Medivir
Medivir is an emerging research-based pharmaceutical company focused on infectious diseases. Medivir has world class expertise in polymerase and protease drug targets and drug development which has resulted in a strong infectious disease R&D portfolio. The Company’s key pipeline asset is simeprevir, a novel protease inhibitor for the treatment of hepatitis C that is being developed in collaboration with Janssen R&D Ireland. The company is also working with research and development in other areas, such as bone disorders and neuropathic pain. Medivir has also a broad product portfolio with prescription pharmaceuticals in the Nordics.
No comments:
Post a Comment